Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0079) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
HMR-1275
|
|||||
| Synonyms |
Alvocidib; Flavopiridol; Flavopiridol (Alvocidib); Flavopiridol hydrochloride; L 86-8275; L 868275; L-868275; L86-8275; 146426-40-6; 2-(2-CHLORO-PHENYL)-5,7-DIHYDROXY-8-(3-HYDROXY-1-METHYL-PIPERIDIN-4-YL)-4H-BENZOPYRAN-4-ONE; 2-(2-chlorophenyl)-5,7-dihydroxy-8-((3S,4R)-3-hydroxy-1-methylpiperidin-4-yl)-4H-chromen-4-one; 2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3S,4R)-3-hydroxy-1-methylpiperidin-4-yl]-4H-chromen-4-one; 45AD6X575G; CHEMBL428690; FLAVO; HMR 1274; HMR 1275; HMR-1275; UNII-45AD6X575G
|
|||||
| Indication | Chronic lymphocytic leukaemia [ICD11: 2A82] | Phase 2 | [1] | |||
| Structure |
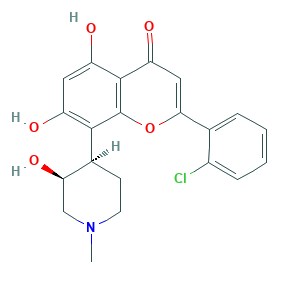 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 401.8 | Topological Polar Surface Area | 90.2 | ||
| Heavy Atom Count | 28 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 6 | |||
| Cross-matching ID |
|
|||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

