| Synonyms |
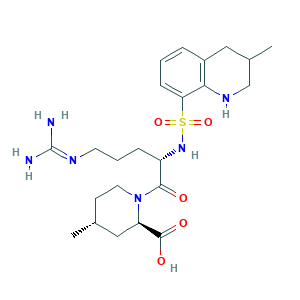
Argatroban Injection; Argatroban [INN:JAN]; Argatroban anhydrous; Argatrobanum [Latin]; DK-7419; Argatroban In Sodium Chloride; GN-1600; MCI 9038; MCI-9038; MD 805; MD-805; Novastan; Slonnon; argatroban; (2R,4R)-1-((2S)-5-Guanidino-2-(3-methyl-1,2,3,4-tetrahydroquinoline-8-sulfonamido)pentanoyl)-4-methylpiperidine-2-carboxylic acid; (2R,4R)-1-[(2S)-5-(diaminomethylideneamino)-2-[(3-methyl-1,2,3,4-tetrahydroquinolin-8-yl)sulfonylamino]pentanoyl]-4-methylpiperidine-2-carboxylic acid; 74863-84-6; Acova; C23H36N6O5S; CHEMBL1166
|
| Cross-matching ID |
- PubChem CID
- 92722
- PubChem SID
-
14786817
; 14786818
; 26719855
; 44423523
; 46386718
; 49681688
; 50015606
; 50111795
; 76329972
; 87225383
; 92308497
; 92309228
; 103072462
; 103079898
; 103339406
; 103945452
; 104408187
; 117541869
; 123120899
; 124658931
; 125311690
; 126664536
; 128449126
; 135062360
; 136379666
; 152059031
; 152212228
; 160814640
; 162011683
; 162178885
; 162254934
; 163089948
; 163670117
; 164788049
; 172080306
; 174006836
; 174473281
; 174530566
; 175607554
; 176484535
; 178103001
; 179116564
; 179295943
; 179323694
; 184643919
; 184816446
; 198993723
; 203355751
; 204433880
; 210279520
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D07UWV
- Formula
- C23H36N6O5S
- Canonical SMILES
- CC1CCN(C(C1)C(=O)O)C(=O)C(CCCN=C(N)N)NS(=O)(=O)C2=CC=CC3=C2NCC(C3)C
- InChI
- 1S/C23H36N6O5S/c1-14-8-10-29(18(12-14)22(31)32)21(30)17(6-4-9-26-23(24)25)28-35(33,34)19-7-3-5-16-11-15(2)13-27-20(16)19/h3,5,7,14-15,17-18,27-28H,4,6,8-13H2,1-2H3,(H,31,32)(H4,24,25,26)/t14-,15?,17+,18-/m1/s1
- InChIKey
- KXNPVXPOPUZYGB-IOVMHBDKSA-N
|