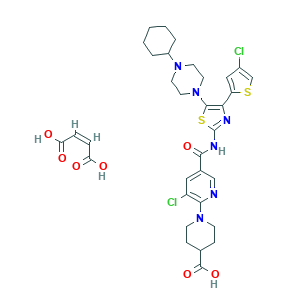
| Synonyms |
Avatrombopag maleate; Avatrombopag maleate (JAN/USAN); Avatrombopag maleate [USAN]; CHEMBL2105758; D10307; Doptelet (TN); E-5501; E5501 MALEATE; GDW7M2P1IS; SCHEMBL19610454; UNII-GDW7M2P1IS; YM-477; 4-Piperidinecarboxylic acid, 1-(3-chloro-5-(((4-(4-chloro-2-thienyl)-5-(4-cyclohexyl-1- piperazinyl)-2-thiazolyl)amino)carbonyl)-2-pyridinyl)-, (2Z)-2-butenedioate (1:1); 677007-74-8; AKR-501 monomaleate
|
| Cross-matching ID |
- PubChem CID
- 9918581
- PubChem SID
-
14889047
; 24196785
; 45990222
; 78363911
; 136349980
; 160692703
; 162108666
; 163312338
; 198978504
- CAS Number
-
- TTD Drug ID
- D0Z5AV
- Formula
- C33H38Cl2N6O7S2
- Canonical SMILES
- C1CCC(CC1)N2CCN(CC2)C3=C(N=C(S3)NC(=O)C4=CC(=C(N=C4)N5CCC(CC5)C(=O)O)Cl)C6=CC(=CS6)Cl.C(=CC(=O)O)C(=O)O
- InChI
- 1S/C29H34Cl2N6O3S2.C4H4O4/c30-20-15-23(41-17-20)24-27(37-12-10-35(11-13-37)21-4-2-1-3-5-21)42-29(33-24)34-26(38)19-14-22(31)25(32-16-19)36-8-6-18(7-9-36)28(39)40;5-3(6)1-2-4(7)8/h14-18,21H,1-13H2,(H,39,40)(H,33,34,38);1-2H,(H,5,6)(H,7,8)/b;2-1-
- InChIKey
- MISPBGHDNZYFNM-BTJKTKAUSA-N
|