| Synonyms |
Bexaroten; Bexarotene; LG 100069; LG 1069; LG-100069; LG100069; LGD 1069; LGD-1069; LGD1069; Targret; Targretin; Targretyn; Targrexin; bexarotenum; 153559-49-0; 166175-31-1; 4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaphthalen-2-yl)ethenyl]benzoic acid; 4-[1-(3,5,5,8,8-pentamethyl-6,7-dihydronaphthalen-2-yl)ethenyl]benzoic acid; A61RXM4375; CHEBI:50859; CHEMBL1023; HSDB 7453; MFCD00932428; UNII-A61RXM4375; p-(1-(5,6,7,8-Tetrahydro-3,5,5,8,8-pentamethyl-2-naphthyl)vinyl)benzoic acid
|
| Cross-matching ID |
- PubChem CID
- 82146
- PubChem SID
-
7978490
; 10219206
; 11528625
; 14876119
; 17397261
; 43137306
; 46509119
; 50125754
; 50156197
; 53790324
; 56310969
; 56313259
; 56313930
; 56352964
; 57288573
; 57333349
; 74382986
; 85246146
; 87550948
; 92308910
; 93167039
; 103274364
; 103941130
; 104379861
; 118314715
; 124893632
; 124893633
; 125341807
; 126591248
; 126646347
; 126667092
; 127324027
; 127324028
; 127324029
; 127324030
; 127324031
; 127324032
; 127324033
; 128675752
; 131300153
; 131407703
; 134220606
; 134337590
; 135037531
; 135650520
; 135692419
; 136340346
; 136946490
; 137002663
; 142405765
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0N0RU
- Formula
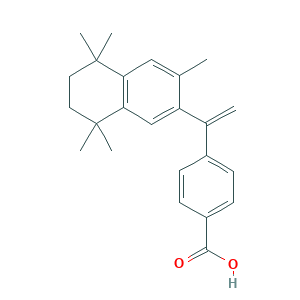
- C24H28O2
- Canonical SMILES
- CC1=CC2=C(C=C1C(=C)C3=CC=C(C=C3)C(=O)O)C(CCC2(C)C)(C)C
- InChI
- 1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26)
- InChIKey
- NAVMQTYZDKMPEU-UHFFFAOYSA-N
|