| Synonyms |
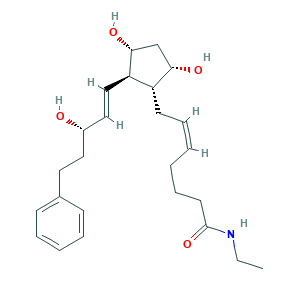
Bimatoprost; LS-181817; Latisse; Lumigan; Lumigan (TN); QXS94885MZ; bimatoprostum; (Z)-7-((1R,2R,3R,5S)-3,5-Dihydroxy-2-((1E,3S)-3-hydroxy-5-phenyl-1-pentenyl)cyclopentyl)-N-ethyl-5-heptenamide; (Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(E,3S)-3-hydroxy-5-phenylpent-1-enyl]cyclopentyl]-N-ethylhept-5-enamide; 155206-00-1; 5-Heptenamide, 7-(3,5-dihydroxy-2-(3-hydrdoxy-5-phenyl-1-pentenyl)cyclopentyl)-N-ethyl-, (1R-(1alpha(Z),2beta(1E,3S*),3alpha,5alpha))-; AC1NSJUW; AGN 192024; AGN-192024; CHEBI:51230; UNII-QXS94885MZ
|
| Cross-matching ID |
- PubChem CID
- 5311027
- PubChem SID
-
7978793
; 11056218
; 11528907
; 12015127
; 14855657
; 14880018
; 17396891
; 17403938
; 39340757
; 46505334
; 50126297
; 50464908
; 56464316
; 71851420
; 90342416
; 90342418
; 99443334
; 103770946
; 114155088
; 126592972
; 126666993
; 134223006
; 134338457
; 135256698
; 135649995
; 137005798
; 142063913
; 152134985
; 152344136
; 160964244
; 162180462
; 174548912
; 175266561
; 175437834
; 179150000
; 210279322
; 210281645
; 223392784
; 223660297
; 223704349
; 224493205
; 226412773
; 250184579
; 251912536
; 251915515
; 252214992
; 252448483
; 252822160
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0Q2XF
- Formula
- C25H37NO4
- Canonical SMILES
- CCNC(=O)CCCC=CCC1C(CC(C1C=CC(CCC2=CC=CC=C2)O)O)O
- InChI
- 1S/C25H37NO4/c1-2-26-25(30)13-9-4-3-8-12-21-22(24(29)18-23(21)28)17-16-20(27)15-14-19-10-6-5-7-11-19/h3,5-8,10-11,16-17,20-24,27-29H,2,4,9,12-15,18H2,1H3,(H,26,30)/b8-3-,17-16+/t20-,21+,22+,23-,24+/m0/s1
- InChIKey
- AQOKCDNYWBIDND-FTOWTWDKSA-N
|