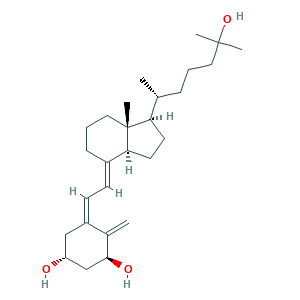
| Synonyms |
Calcijex; Calcitriolum; Calcitriolum [INN-Latin]; DN 101; DN-101; Dihydroxyvitamin D3; Ro 21-5535; Rocaltrol; Silkis; Soltriol; Topitriol; Vectical; calcitriol; 1,25-DHCC; 1,25-DIHYDROXYCHOLECALCIFEROL; 1,25-Dihydroxycholecaliferol; 1,25-Dihydroxyvitamin D; 1,25-Dihydroxyvitamin D3; 1-alpha,25-Dihydroxycholecalciferol; 1-alpha,25-Dihydroxyvitamin D3; 1alpha,25(OH)2D3; 1alpha,25-Dihydroxycholecalciferol; 1alpha,25-Dihydroxyvitamin D; 1alpha,25-Dihydroxyvitamin D3; 32222-06-3; CCRIS 5522; EINECS 250-963-8; HSDB 3482; UNII-FXC9231JVH
|
| Cross-matching ID |
- PubChem CID
- 5280453
- PubChem SID
-
4817
; 596357
; 628349
; 646010
; 3225659
; 7847197
; 7850823
; 7978847
; 8143990
; 8616278
; 11528639
; 12012574
; 14758022
; 14855739
; 24850808
; 24893473
; 26719902
; 26754891
; 26759742
; 39289574
; 46386784
; 46391717
; 46508162
; 47588739
; 47662005
; 47959455
; 48334202
; 48415682
; 49681438
; 49681793
; 50110818
; 53789169
; 53789350
; 56311224
; 56311227
; 56311279
; 56311508
; 56311775
; 56312928
; 56313898
; 56314589
; 56365636
; 57288572
; 57357744
; 57647688
; 71824963
; 80321599
; 89088644
; 92308336
; 92308939
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0T2PL
- Formula
- C27H44O3
- Canonical SMILES
- CC(CCCC(C)(C)O)C1CCC2C1(CCCC2=CC=C3CC(CC(C3=C)O)O)C
- InChI
- 1S/C27H44O3/c1-18(8-6-14-26(3,4)30)23-12-13-24-20(9-7-15-27(23,24)5)10-11-21-16-22(28)17-25(29)19(21)2/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b20-10+,21-11-/t18-,22-,23-,24+,25+,27-/m1/s1
- InChIKey
- GMRQFYUYWCNGIN-NKMMMXOESA-N
|