Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0272) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Carboplatin
|
|||||
| Synonyms |
Carboplatin; Paraplatin; RL03611; 1,1-Cyclobutanedicarboxylatodiammineplatinum(II); 10329-EP2281815A1; 10329-EP2292617A1; 10329-EP2298765A1; 10329-EP2301538A1; 10329-EP2305243A1; 10329-EP2308812A2; 10329-EP2308833A2; 10329-EP2308839A1; 10329-EP2311455A1; 10329-EP2311807A1; 10329-EP2311825A1; 10329-EP2314590A1; 10329-EP2316833A1; 41575-94-4; AB01568249_01; BG3F62OND5; Cbdca; JM-8; NSC 241240; UNII-BG3F62OND5; cis-Diammine(1,1-cyclobutanedicarboxylato) platinum; cis-Diammine(1,1-cyclobutanedicarboxylato)platinum(II); s1215
|
|||||
| Indication | Ovarian cancer [ICD11: 2C73] | Approved | [1] | |||
| Structure |
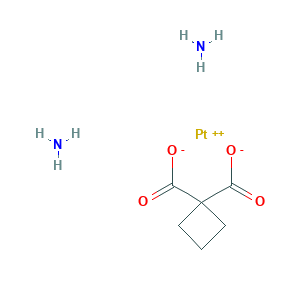 |
|||||
| 3D MOL is unavailable | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 371.25 | Topological Polar Surface Area | 82.3 | ||
| Heavy Atom Count | 13 | Rotatable Bond Count | 0 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 6 | |||
| Cross-matching ID |
|
|||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

