| General Information of Drug (ID:
DR0285) |
| Drug Name |
Cefaloridine
|
| Synonyms |
Cefaloridin; Cefaloridina; Cefaloridina [INN-Spanish]; Cefaloridinum; Cefaloridinum [INN-Latin]; Cefalorizin; Ceflorin; Cepaloridin; Cepalorin; Cephalomycine; Cephaloridin; Cephaloridinum; Ceporan; Ceporin; Ceporine; Cilifor; Deflorin; Ampligram; Betaine cephaloridine; Faredina; Glaxoridin; Intrasporin; Keflodin; Keflordin; Kefloridin; Kefspor; Lloncefal; Loridine; Sasperin; Sefacin; Verolgin; Vioviantine; cefaloridine; cephaloridine; 50-59-9; CHEBI:3537; N-(7-(2'-Thienylacetamidoceph-3-ylmethyl))-pyridinium-2-carboxylate; UNII-LVZ1VC61HB
|
| Indication |
Staphylococcus infection
[ICD11: 1B73]
|
Phase 4
|
[1]
|
| Structure |
|
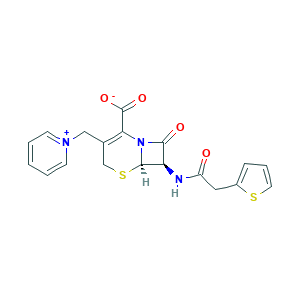 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
415.5 |
Topological Polar Surface Area |
147 |
| Heavy Atom Count |
28 |
Rotatable Bond Count |
5 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
6 |
| Cross-matching ID |
- PubChem CID
- 5773
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0F8IO
- Formula
- C19H17N3O4S2
- Canonical SMILES
- C1C(=C(N2C(S1)C(C2=O)NC(=O)CC3=CC=CS3)C(=O)[O-])C[N+]4=CC=CC=C4
- InChI
- 1S/C19H17N3O4S2/c23-14(9-13-5-4-8-27-13)20-15-17(24)22-16(19(25)26)12(11-28-18(15)22)10-21-6-2-1-3-7-21/h1-8,15,18H,9-11H2,(H-,20,23,25,26)/t15-,18-/m1/s1
- InChIKey
- CZTQZXZIADLWOZ-CRAIPNDOSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


