| General Information of Drug (ID:
DR0304) |
| Drug Name |
Chlorpheniramine
|
| Synonyms |
Chlorphenamine, (R)-; Chlorpheniramine, (-)-; Chlorpheniramine, (R)-; EY9131E63D; Lopac-C-3025; CHLORPHENIRAMINE (S) MALEATE; Lopac-C-4915; SCHEMBL4220; SPBio_000292; Spectrum2_000156; Spectrum3_000345; Spectrum4_000282; Spectrum5_001442; Spectrum_000140; l-chlorpheniramine; levochlorpheniramine; (-)-Chloropheniramine; (-)-chlorpheniramine; (3R)-3-(4-chlorophenyl)-N,N-dimethyl-3-pyridin-2-ylpropan-1-amine; 32188-09-3; AC1LEQ0Y; BSPBio_002009; CAS-113-92-8; CHEBI:52013; GTPL1213; KBioGR_000804; KBioSS_000600; UNII-EY9131E63D
|
| Indication |
Allergic rhinitis
[ICD11: CA08]
|
Approved
|
[1]
|
| Structure |
|
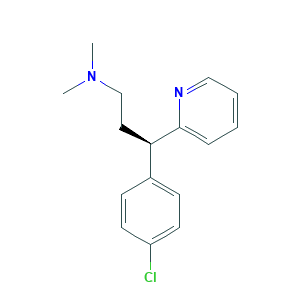 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
274.79 |
Topological Polar Surface Area |
16.1 |
| Heavy Atom Count |
19 |
Rotatable Bond Count |
5 |
| Hydrogen Bond Donor Count |
0 |
Hydrogen Bond Acceptor Count |
2 |
| Cross-matching ID |
- PubChem CID
- 716121
- PubChem SID
-
9299815
; 11110931
; 11110932
; 11112256
; 11363014
; 11365576
; 11368138
; 11371329
; 11373943
; 11376300
; 11484028
; 11487972
; 11490161
; 11492097
; 11493954
; 15442268
; 33774101
; 47719847
; 48018097
; 50064683
; 50100400
; 50225325
; 57304992
; 85789162
; 109896795
; 135650086
; 135774423
; 137002507
; 160666558
; 174007153
; 175269603
; 179039539
; 226396260
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0B7NG
- Formula
- C16H19ClN2
- Canonical SMILES
- CN(C)CCC(C1=CC=C(C=C1)Cl)C2=CC=CC=N2
- InChI
- 1S/C16H19ClN2/c1-19(2)12-10-15(16-5-3-4-11-18-16)13-6-8-14(17)9-7-13/h3-9,11,15H,10,12H2,1-2H3/t15-/m1/s1
- InChIKey
- SOYKEARSMXGVTM-OAHLLOKOSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


