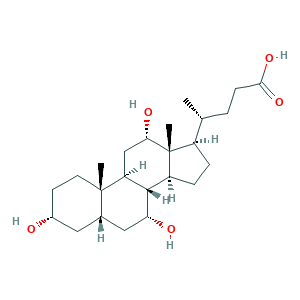
| Synonyms |
Cholalic acid; Cholalin; Cholbam; Cholic acid [USAN]; Cholic acid, 5beta-; Cholsaeure; Colalin; cholate; cholic acid; (R)-4-((3R,5S,7R,8R,9S,10S,12S,13R,14S,17R)-3,7,12-Trihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoic acid; 3,7,12-Trihydroxycholanic acid; 3alpha,7alpha,12alpha-Trihydroxy-5beta-cholan-24-oic acid; 3alpha,7alpha,12alpha-Trihydroxy-5beta-cholanic acid; 81-25-4; CHEBI:16359; CHEMBL205596; G1JO7801AE; HSDB 982; MFCD00003672; NSC-6135; NSC6135; UNII-G1JO7801AE
|
| Cross-matching ID |
- PubChem CID
- 221493
- PubChem SID
-
3963
; 72129
; 584143
; 607109
; 822057
; 841391
; 3135999
; 4266387
; 7886587
; 8145669
; 9376190
; 11110315
; 14757556
; 15277848
; 17422064
; 24423691
; 24423929
; 24892356
; 24893153
; 26717723
; 26717726
; 26717727
; 26737120
; 26737126
; 26737128
; 26750105
; 26750407
; 26750412
; 26750415
; 26750420
; 26750424
; 30424718
; 46507063
; 47193710
; 47662491
; 49700787
; 50452100
; 53787750
; 53789207
; 56313736
; 56437567
; 56437568
; 57399957
; 81067251
; 81091088
; 85279435
; 85285851
; 85285861
; 85285865
; 85285871
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0OR2L
- Formula
- C24H40O5
- Canonical SMILES
- CC(CCC(=O)O)C1CCC2C1(C(CC3C2C(CC4C3(CCC(C4)O)C)O)O)C
- InChI
- 1S/C24H40O5/c1-13(4-7-21(28)29)16-5-6-17-22-18(12-20(27)24(16,17)3)23(2)9-8-15(25)10-14(23)11-19(22)26/h13-20,22,25-27H,4-12H2,1-3H3,(H,28,29)/t13-,14+,15-,16-,17+,18+,19-,20+,22+,23+,24-/m1/s1
- InChIKey
- BHQCQFFYRZLCQQ-OELDTZBJSA-N
|