| Synonyms |
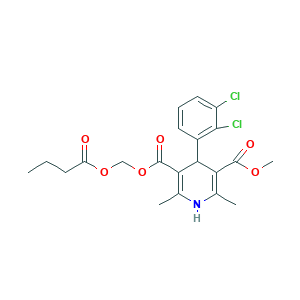
Clevelox; Clevidipine; Clevidipine butyrate; Cleviprex; rac-Clevidipine; 167221-71-8; 5-O-(butanoyloxymethyl) 3-O-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate; H 324/38; Methyl (1-oxobutoxy)methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dime thyl-3,5-pyridinedicarboxylate; Methyl (1-oxobutoxy)methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate
|
| Cross-matching ID |
- PubChem CID
- 153994
- PubChem SID
-
10261840
; 33514704
; 50580186
; 103066579
; 103084376
; 113457368
; 117583516
; 121632362
; 137185876
; 138382287
; 175271058
; 184547275
; 226487864
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D09ELP
- Formula
- C21H23Cl2NO6
- Canonical SMILES
- CCCC(=O)OCOC(=O)C1=C(NC(=C(C1C2=C(C(=CC=C2)Cl)Cl)C(=O)OC)C)C
- InChI
- 1S/C21H23Cl2NO6/c1-5-7-15(25)29-10-30-21(27)17-12(3)24-11(2)16(20(26)28-4)18(17)13-8-6-9-14(22)19(13)23/h6,8-9,18,24H,5,7,10H2,1-4H3
- InChIKey
- KPBZROQVTHLCDU-UHFFFAOYSA-N
|