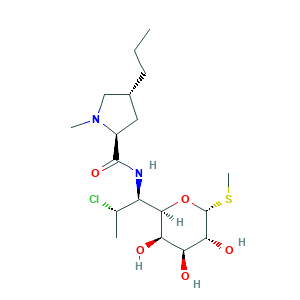
| Synonyms |
Antirobe; Chlolincocin; ClindaDerm; Clindamicina; Clindamycine; Clindamycinum; Clinimycin; Dalacin C; Dalacine; Klimicin; Klindan 300; Sobelin; U 21251; U-21251; clindamycin; 18323-44-9; 3U02EL437C; 7(S)-Chloro-7-deoxylincomycin; 7-CDL; 7-Chloro-7-deoxylincomycin; 7-Chlorolincomycin; 7-Deoxy-7(S)-chlorolincomycin; C18H33ClN2O5S; EINECS 242-209-1; HSDB 3037; UNII-3U02EL437C; methyl 7-chloro-6,7,8-trideoxy-6-{[(4R)-1-methyl-4-propyl-L-prolyl]amino}-1-thio-L-threo-alpha-D-galacto-octopyranoside
|
| Cross-matching ID |
- PubChem CID
- 446598
- CAS Number
-
- TTD Drug ID
- D0R0ZL
- Formula
- C18H33ClN2O5S
- Canonical SMILES
- CCCC1CC(N(C1)C)C(=O)NC(C2C(C(C(C(O2)SC)O)O)O)C(C)Cl
- InChI
- 1S/C18H33ClN2O5S/c1-5-6-10-7-11(21(3)8-10)17(25)20-12(9(2)19)16-14(23)13(22)15(24)18(26-16)27-4/h9-16,18,22-24H,5-8H2,1-4H3,(H,20,25)/t9-,10+,11-,12+,13-,14+,15+,16+,18+/m0/s1
- InChIKey
- KDLRVYVGXIQJDK-AWPVFWJPSA-N
|