| Synonyms |
Cortadren; Cortelan; Cortilen; Cortisone (acetate); Cortisone 21-acetate; Cortisone monoacetate; Cortisone, 21-acetate; Cortistab; Cortisyl; Cortogen acetate; Cortone acetate; EINECS 200-006-5; Incortin; Irisone acetate; Cortandren; Cortisone; Cortisal; Cortisate; Cortison; Cortisona; Cortisona [INN-Spanish]; Cortisone [INN:BAN]; Cortisonum [INN-Latin]; Cortistal; Cortivite; Cortogen; Cortone; Kendall's compound E; Reichstein Fa; Reichstein's substance FA; Wintersteiner's compound F; 11-Dehydro-17-hydroxycorticosterone; 17-Hydroxy-11-dehydrocorticosterone; 17alpha,21-Dihydroxypregn-4-ene-3,11,20-trione; 17alpha-Hydroxy-11-dehydrocorticosterone; 1953/6/5; 4-Pregnene-17alpha,21-diol-3,11,20-trione; Adrenalex; Andreson; NSC 49420; Pregn-4-ene-3,11,20-trione, 21-(acetyloxy)-17-hydroxy-; Ricortex; Scheroson; UNII-883WKN7W8X; 11-Dehydro-17-hydroxycorticosterone acetate; 11-Dehydro-17-hydroxycorticosterone-21-acetate; 1950/4/4; Adreson; Artriona; Biocort acetate; CCRIS 3661; CORTISONE ACETATE; Compound E acetate; Corlin
|
| Cross-matching ID |
- PubChem CID
- 5745
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0X4RS
- Formula
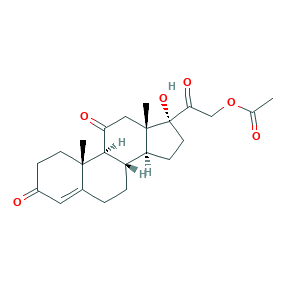
- C23H30O6
- Canonical SMILES
- CC(=O)OCC(=O)C1(CCC2C1(CC(=O)C3C2CCC4=CC(=O)CCC34C)C)O
- InChI
- 1S/C23H30O6/c1-13(24)29-12-19(27)23(28)9-7-17-16-5-4-14-10-15(25)6-8-21(14,2)20(16)18(26)11-22(17,23)3/h10,16-17,20,28H,4-9,11-12H2,1-3H3/t16-,17-,20+,21-,22-,23-/m0/s1
- InChIKey
- ITRJWOMZKQRYTA-RFZYENFJSA-N
|