Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0395) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Cyproheptadine
|
|||||
| Synonyms |
Ciproheptadina; Ciproheptadina [INN-Spanish]; Cypoheptadine; Cyproheptadiene; Cyproheptadine [INN:BAN]; Cyproheptadinum; Cyproheptadinum [INN-Latin]; Dihexazin; Dronactin; Eiproheptadine; MK 141; Periactin; Periactine; Periactinol; CYPROHEPTADINE; Peritol; 1-Methyl-4-(5-dibenzo(a,e)cycloheptatrienylidene)piperidine; 1-Methyl-4-(5H-dibenzo(a,d)cycloheptenylidene)piperidine; 129-03-3; 4-(5H-dibenzo[a,d][7]annulen-5-ylidene)-1-methylpiperidine; 5-(1-Methylpiperidylidene-4)-5H-dibenzo(a,d)cyclopheptene
|
|||||
| Indication | Rheumatoid arthritis [ICD11: FA20] | Approved | [1] | |||
| Structure |
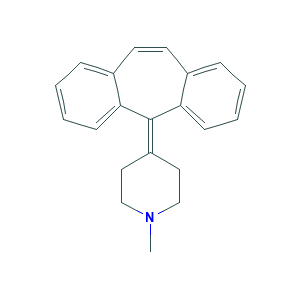 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 287.4 | Topological Polar Surface Area | 3.2 | ||
| Heavy Atom Count | 22 | Rotatable Bond Count | 0 | |||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 1 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

