| Cross-matching ID |
- PubChem CID
- 6253
- PubChem SID
-
5877
; 598052
; 829245
; 3260118
; 7847236
; 7885952
; 7979015
; 8153933
; 11528303
; 11533128
; 12146044
; 14774115
; 15196510
; 24858315
; 24892435
; 26527898
; 26719755
; 29215144
; 29225249
; 46386862
; 46505879
; 47193873
; 47573376
; 48415837
; 49831046
; 50105698
; 53787662
; 56310997
; 56311029
; 56312269
; 56312943
; 56313129
; 56313612
; 76890970
; 83110442
; 87558791
; 90341066
; 92308449
; 92309014
; 93166529
; 93167157
; 103210760
; 103986361
; 104253284
; 104311532
; 117529237
; 118048876
; 124648720
; 124757405
; 124799562
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D07XSN
- Formula
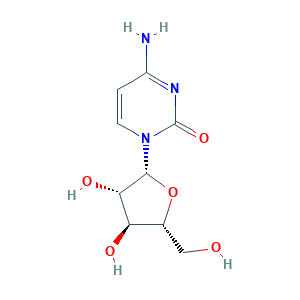
- C9H13N3O5
- Canonical SMILES
- C1=CN(C(=O)N=C1N)C2C(C(C(O2)CO)O)O
- InChI
- 1S/C9H13N3O5/c10-5-1-2-12(9(16)11-5)8-7(15)6(14)4(3-13)17-8/h1-2,4,6-8,13-15H,3H2,(H2,10,11,16)/t4-,6-,7+,8-/m1/s1
- InChIKey
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N
|