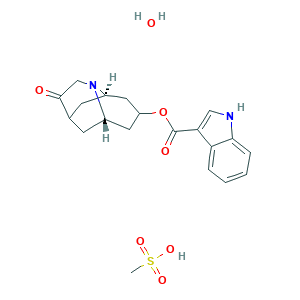
| Synonyms |
Dalasetron (Mesylate hydrate); Dalasetron Mesylate Hydrate; Dolasetron (Mesylate hydrate); Dolasetron mesilate; Dolasetron mesylate hydrate; Dolasetron methanesulfonate hydrate; H668; HMS3714B12; HY-B0750B; MDL-73147EF; NCGC00181048-01; QTFFGPOXNNGTGZ-LIFGOUTFSA-N; SCHEMBL1237588; Dolasetron; Dolasetronum; Dolasteron; HY-B0750; SCHEMBL42063; SCHEMBL42064; UKTAZPQNNNJVKR-KJGYPYNMSA-N; ZINC2688; (2,6,8,9a)-octahydro-3-oxo-2,6-methano-2H-quinolizin-8-yl-1Hindole-3-carboxylate monomethanesulfonate, monohydrate; (2alpha,6alpha,8alpha,9abeta)-octahydro-3-oxo-2,6-methano-2h-quinolizin-8-yl-1h-indole-3-carboxylate; 115956-12-2; 1H-Indole-3-carboxylic acid, octahydro-3-oxo-2,6-methano-2H-quinolizin-8-yl ester, stereoisomer; 3594AH; AC1MHWDD; Anzemet Cinv; BIDD:GT0287; CHEBI:94561; CHEMBL2368925; DTXSID4048276; 115956-13-3; 878143-33-0; 956D133; API0002473; AS-13228; Anemet; Anzemet; Anzemet hydrate; C19H20N2O3.CH4O3S.H2O; CCG-220885; CCG-222446; CHEMBL2368924; CS-3746; DOLASETRON MESYLATE; DOLASETRON MESYLATE MONOHYDRATE
|
| Cross-matching ID |
- PubChem CID
- 6918119
- CAS Number
-
- TTD Drug ID
- D00YLW
- Formula
- C20H26N2O7S
- Canonical SMILES
- CS(=O)(=O)O.C1C2CC3CC(CC1N3CC2=O)OC(=O)C4=CNC5=CC=CC=C54.O
- InChI
- 1S/C19H20N2O3.CH4O3S.H2O/c22-18-10-21-12-5-11(18)6-13(21)8-14(7-12)24-19(23)16-9-20-17-4-2-1-3-15(16)17;1-5(2,3)4;/h1-4,9,11-14,20H,5-8,10H2;1H3,(H,2,3,4);1H2/t11?,12-,13+,14?;;
- InChIKey
- QTFFGPOXNNGTGZ-RCSCTSIBSA-N
|