| Synonyms |
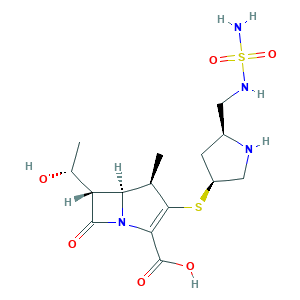
Doribax; Doripenem; Doripenem hydrate; Doripenem monohydrate; Finibax; S-4661; BHV525JOBH; (4R,5S,6S)-6-((R)-1-hydroxyethyl)-4-methyl-7-oxo-3-(((3S,5S)-5-((sulfamoylamino)methyl)pyrrolidin-3-yl)thio)-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid; (4R,5S,6S)-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-3-[(3S,5S)-5-[(sulfamoylamino)methyl]pyrrolidin-3-yl]sulfanyl-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid; 148016-81-3; DRPM; DSSTox_CID_26678; DSSTox_GSID_46678; DSSTox_RID_81814; NCGC00167510-01; UNII-BHV525JOBH
|
| Cross-matching ID |
- PubChem CID
- 73303
- PubChem SID
-
8195661
; 14782518
; 14904822
; 17397979
; 43129277
; 50112763
; 57319033
; 78931074
; 99299161
; 103596434
; 104354952
; 118047125
; 126665586
; 134339262
; 135030316
; 136367963
; 137103545
; 140972593
; 144115919
; 144206061
; 152057781
; 152344294
; 164824831
; 170465085
; 174527594
; 175266776
; 175437740
; 179117027
; 184547713
; 210279313
; 210281636
; 223556613
; 223659794
; 226423250
; 241037251
; 242439235
; 249358056
; 249583208
; 249822520
; 251963975
; 251970995
; 252215304
; 252348782
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D03QWT
- Formula
- C15H24N4O6S2
- Canonical SMILES
- CC1C2C(C(=O)N2C(=C1SC3CC(NC3)CNS(=O)(=O)N)C(=O)O)C(C)O
- InChI
- 1S/C15H24N4O6S2/c1-6-11-10(7(2)20)14(21)19(11)12(15(22)23)13(6)26-9-3-8(17-5-9)4-18-27(16,24)25/h6-11,17-18,20H,3-5H2,1-2H3,(H,22,23)(H2,16,24,25)/t6-,7-,8+,9+,10-,11-/m1/s1
- InChIKey
- AVAACINZEOAHHE-VFZPANTDSA-N
|