| General Information of Drug (ID:
DR0577) |
| Drug Name |
Enclomiphene
|
| Synonyms |
Enclomifene; Enclomifeno; Enclomifeno [INN-Spanish]; Enclomifenum; Enclomifenum [INN-Latin]; Enclomiphene; Enclomiphene (USAN); Enclomiphene [USAN]; GKIRPKYJQBWNGO-OCEACIFDSA-N; ISOMER B; R6D2UI4FLS; RMI 16,289; Transclomifenum; Cisclomiphene; Clomifene; Clomifeno; Transclomiphene; clomiphene; trans-Clomifene; trans-Clomiphene; (E)-2-(p-(2-Chloro-1,2-diphenylvinyl)phenoxy)triethylamine; 15690-57-0; 911-45-5; ICI 46476; UNII-R6D2UI4FLS; trans-2-(p-(2-Chloro-1,2-diphenylvinyl)phenoxy)triethylamine
|
| Indication |
Female infertility
[ICD11: GA31]
|
Phase 4
|
[1]
|
| Structure |
|
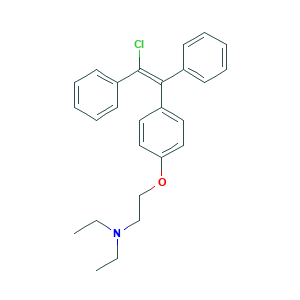 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
406 |
Topological Polar Surface Area |
12.5 |
| Heavy Atom Count |
29 |
Rotatable Bond Count |
9 |
| Hydrogen Bond Donor Count |
0 |
Hydrogen Bond Acceptor Count |
2 |
| Cross-matching ID |
- PubChem CID
- 1548953
- PubChem SID
-
9134
; 7978971
; 7979159
; 8653308
; 14879465
; 32248894
; 46504463
; 47646665
; 48094648
; 48169503
; 48169504
; 49698470
; 50058156
; 50085975
; 50124218
; 51092022
; 56352921
; 57409058
; 75735317
; 81093254
; 85788573
; 90452382
; 92309293
; 93165675
; 93166374
; 96025593
; 103249489
; 104057024
; 110525086
; 117721819
; 124800080
; 124893612
; 126690411
; 131742069
; 135024045
; 139650876
; 164235770
; 175270141
; 176484319
; 179148468
; 184022027
; 198962245
; 223365954
; 224924849
; 250133940
; 251960682
; 252635354
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0CT9Y
- Formula
- C26H28ClNO
- Canonical SMILES
- CCN(CC)CCOC1=CC=C(C=C1)C(=C(C2=CC=CC=C2)Cl)C3=CC=CC=C3
- InChI
- 1S/C26H28ClNO/c1-3-28(4-2)19-20-29-24-17-15-22(16-18-24)25(21-11-7-5-8-12-21)26(27)23-13-9-6-10-14-23/h5-18H,3-4,19-20H2,1-2H3/b26-25+
- InChIKey
- GKIRPKYJQBWNGO-OCEACIFDSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


