| General Information of Drug (ID:
DR0586) |
| Drug Name |
Ephedrin
|
| Synonyms |
Eciphin; Efedrin; Ephedral; Ephedremal; Ephedrin; Ephedrine; Ephedrine [USAN:BAN]; Ephedrine l-form; Ephedrital; Ephedrol; Ephedrosan; Ephedrotal; Ephedsol; Ephendronal; Ephoxamin; Fedrin; I-Sedrin; Kratedyn; L(-)-Ephedrine; Biophedrin; Lexofedrin; Manadrin; Mandrin; Norephedrine, N-methyl-; Sanedrine; Vencipon; Zephrol; l-Ephedrine; racephedrine; (-)-Ephedrine; (1R,2S)-2-(methylamino)-1-phenylpropan-1-ol; (L)-EPHEDRINE; 1-Phenyl-2-methylaminopropanol; 1-Sedrin; 299-42-3; HSDB 3072; Nasol; UNII-GN83C131XS
|
| Indication |
Contraception
[ICD11: JA65]
|
Phase 4
|
[1]
|
| Structure |
|
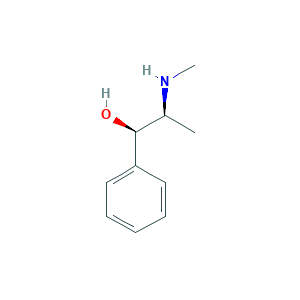 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
165.23 |
Topological Polar Surface Area |
32.299 |
| Heavy Atom Count |
12 |
Rotatable Bond Count |
3 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
2 |
| Cross-matching ID |
- PubChem CID
- 9294
- PubChem SID
-
4732
; 598368
; 7847192
; 7979173
; 8143173
; 8156646
; 10517637
; 15147083
; 15219436
; 24848190
; 24868714
; 29227903
; 46507538
; 47291377
; 47365458
; 47736757
; 48415944
; 48424318
; 49965013
; 50111064
; 57325562
; 57392731
; 79710055
; 99222720
; 103492152
; 104320122
; 117475916
; 119525662
; 124749764
; 124892369
; 128443809
; 131309442
; 134975118
; 135650234
; 136903786
; 137001226
; 142245143
; 160964651
; 163418533
; 163850169
; 175265573
; 179150516
; 198946050
; 223520862
; 223667135
; 223671453
; 223671797
; 223786876
; 226396713
; 241058217
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0LG8E
- Formula
- C10H15NO
- Canonical SMILES
- CC(C(C1=CC=CC=C1)O)NC
- InChI
- 1S/C10H15NO/c1-8(11-2)10(12)9-6-4-3-5-7-9/h3-8,10-12H,1-2H3/t8-,10-/m0/s1
- InChIKey
- KWGRBVOPPLSCSI-WPRPVWTQSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


