| General Information of Drug (ID:
DR0593) |
| Drug Name |
Epoprostenol
|
| Synonyms |
Epoprostenolum; DCR9Z582X0; Epoprostenol [USAN:INN]; Epoprostenolum [INN-Latin]; KAQKFAOMNZTLHT-OZUDYXHBSA-N; KB-IV-24; PGI(sub 2); Prostacyclin I2; Prostaglandin 12; Prostaglandin X; U 53,217; Vasocyclin; epoprostenol; prostacyclin; prostaglandin I2; 35121-78-9; 6,9-alpha-Epoxy-11-alpha,15(S)-dihydroxyprosta-5(Z),13(E)-dien-1-oic acid; 6,9S-epoxy-11R,15S-dihydoxy-5Z,13E-prostadienoic acid; 63859-31-4; BRN 1690090; CHEBI:15552; PG-I2; PGI2; PGX; UNII-DCR9Z582X0
|
| Indication |
Pulmonary hypertension
[ICD11: BB01]
|
Approved
|
[1]
|
| Structure |
|
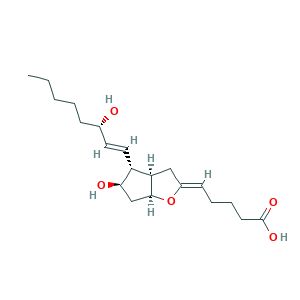 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
352.5 |
Topological Polar Surface Area |
87 |
| Heavy Atom Count |
25 |
Rotatable Bond Count |
10 |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
5 |
| Cross-matching ID |
- PubChem CID
- 5282411
- PubChem SID
-
4266019
; 7979800
; 7980285
; 8143216
; 11038504
; 14900947
; 26754810
; 26754811
; 39315859
; 47291286
; 47662474
; 48110621
; 48415947
; 50110786
; 57358657
; 77700624
; 85788539
; 92309912
; 93166672
; 99300826
; 103326666
; 113857140
; 127327695
; 127327696
; 127335056
; 127335057
; 127335058
; 127335059
; 127335060
; 135000098
; 135651541
; 137003054
; 142047542
; 164756785
; 175267334
; 179150361
; 224259470
; 226399315
; 250134198
; 252216120
; 252378175
; 252455636
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0V0IX
- Formula
- C20H32O5
- Canonical SMILES
- CCCCCC(C=CC1C(CC2C1CC(=CCCCC(=O)O)O2)O)O
- InChI
- 1S/C20H32O5/c1-2-3-4-7-14(21)10-11-16-17-12-15(8-5-6-9-20(23)24)25-19(17)13-18(16)22/h8,10-11,14,16-19,21-22H,2-7,9,12-13H2,1H3,(H,23,24)/b11-10+,15-8-/t14-,16+,17+,18+,19-/m0/s1
- InChIKey
- KAQKFAOMNZTLHT-OZUDYXHBSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


