| Synonyms |
EQUILENIN; Equilenin solution; Equilenina [Spanish]; LMST02010007; SCHEMBL120922; W8FTJ17C4J; ZINC393154; (13S,14S)-3-hydroxy-13-methyl-12,14,15,16-tetrahydro-11H-cyclopenta[a]phenanthren-17-one; 1ogx; 1ogz; 1w6y; 3-Hydroxyestra-1,3,5(10),6,8-pentaen-17-one; 3-Hydroxyoestra-1,3,5(10),6,8-pentaen-17-one; 3-hydroxy-estra-1,3,5(10),6,8-pentaen-17-one; 4-08-00-01420 (Beilstein Handbook Reference); 517-09-9; AC1L9H0U; BDBM50423545; BRN 2335367; CCRIS 9075; CHEMBL225546; CTK8F9475; DTXSID2052156; EINECS 208-230-5; UNII-W8FTJ17C4J
|
| Cross-matching ID |
- PubChem CID
- 444865
- PubChem SID
-
820980
; 828639
; 828643
; 828644
; 836042
; 854746
; 855191
; 7887350
; 10299594
; 14714955
; 15197222
; 15925964
; 17395303
; 24860441
; 26710297
; 36887747
; 46392780
; 46393462
; 46509080
; 53812543
; 57404643
; 57648900
; 74374181
; 79734085
; 81060992
; 87226001
; 87226002
; 92192448
; 103513880
; 104231780
; 104633042
; 119525954
; 125264324
; 125264325
; 125264326
; 125333153
; 125333154
; 126722978
; 126722980
; 128900977
; 134976304
; 137248839
; 160966631
; 163134380
; 164152420
; 176265493
; 179316938
; 198951559
; 223438441
; 223466302
- CAS Number
-
- TTD Drug ID
- D08WDY
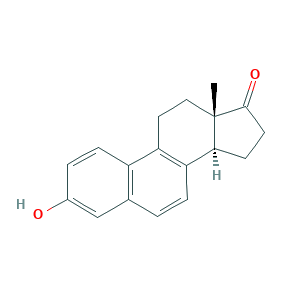
- Formula
- C18H18O2
- Canonical SMILES
- CC12CCC3=C(C1CCC2=O)C=CC4=C3C=CC(=C4)O
- InChI
- 1S/C18H18O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h2-5,10,16,19H,6-9H2,1H3/t16-,18-/m0/s1
- InChIKey
- PDRGHUMCVRDZLQ-WMZOPIPTSA-N
|