| General Information of Drug (ID:
DR0653) |
| Drug Name |
MK-595
|
| Synonyms |
Acide etacrynique; Acide etacrynique [INN-French]; Acido etacrinico; Acido etacrinico [INN-Spanish]; Acidum etacrynicum; Acidum etacrynicum [INN-Latin]; Crinuryl; ETHACRYNIC ACID; Edecril; Edecrin; Edecrina; Endecril; Etacrinic acid; Etacrynic Acid; Etakrinic acid; Ethacrinique (acide); Ethacryinic Acid; Hidromedin; Hydromedin; Kyselina ethakrynova; Kyselina ethakrynova [Czech]; MK-595; Mingit; Otacril; Reomax; Taladren; Uregit; ethacrynate; 58-54-8; Methylenebutyrylphenoxyacetic acid
|
| Indication |
Congenital heart anomaly
[ICD11: LA8Z]
|
Phase 3
|
[1]
|
| Structure |
|
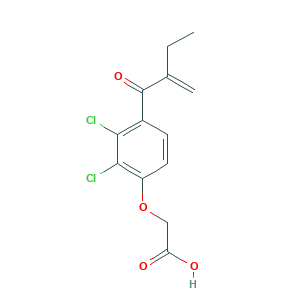 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
303.13 |
Topological Polar Surface Area |
63.6 |
| Heavy Atom Count |
19 |
Rotatable Bond Count |
6 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 3278
- PubChem SID
-
122995
; 491575
; 798369
; 834701
; 855762
; 5208062
; 7847379
; 7887267
; 8149337
; 8152080
; 10321631
; 11112144
; 11336075
; 11361314
; 11363707
; 11366269
; 11368831
; 11371330
; 11374346
; 11376993
; 11446250
; 11462286
; 11466287
; 11467407
; 11483790
; 11486033
; 11487913
; 11490241
; 11492359
; 11494627
; 14776240
; 17389016
; 24894577
; 26611737
; 26680116
; 26706137
; 26746983
; 26746984
; 29222417
; 46392033
; 46394202
; 46507562
; 47662347
; 47736552
; 47959820
; 47959821
; 47959822
; 48110508
; 48185057
; 48423385
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D06TNL
- Formula
- C13H12Cl2O4
- Canonical SMILES
- CCC(=C)C(=O)C1=C(C(=C(C=C1)OCC(=O)O)Cl)Cl
- InChI
- 1S/C13H12Cl2O4/c1-3-7(2)13(18)8-4-5-9(12(15)11(8)14)19-6-10(16)17/h4-5H,2-3,6H2,1H3,(H,16,17)
- InChIKey
- AVOLMBLBETYQHX-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


