| General Information of Drug (ID:
DR0685) |
| Drug Name |
Felodipine
|
| Synonyms |
Feloday; Felodipina; Felodipina [INN-Spanish]; Felodipine (Plendil); Felodipine [USAN:BAN:INN]; Felodipinum; Felodipinum [INN-Latin]; Felodur ER; Felogard; Flodil; Munobal; Munobal Retard; Penedil; Perfudal; Plandil; Plendil; Plendil Depottab; AGON SR; Plendil ER; Plendil Retard; Preslow; Prevex; Renedil; Splendil; dl-Felodipine; felodipine; 3-ethyl 5-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate; 72509-76-3; 86189-69-7; Agon; BRN 4331472; C18H19Cl2NO4; CGH-869; H 154/82; H-154/82; Hydac; MLS000069629; Modip
|
| Indication |
Essential hypertension
[ICD11: BA00]
|
Approved
|
[1]
|
| Structure |
|
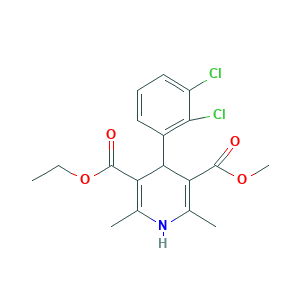 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
384.2 |
Topological Polar Surface Area |
64.599 |
| Heavy Atom Count |
25 |
Rotatable Bond Count |
6 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
5 |
| Cross-matching ID |
- PubChem CID
- 3333
- PubChem SID
-
856004
; 5309057
; 7847385
; 7979219
; 8152117
; 10321744
; 11466506
; 11467626
; 11486291
; 11528719
; 12012613
; 14927301
; 16960569
; 17405071
; 24278449
; 29222468
; 46506968
; 47509540
; 47656605
; 47879634
; 48179244
; 48328566
; 48416001
; 49698512
; 50106289
; 50106290
; 53777614
; 53788382
; 56423042
; 56424130
; 56463217
; 56463516
; 57321731
; 78292296
; 85231057
; 85788575
; 87351806
; 90340828
; 92125582
; 92303867
; 92308861
; 92711325
; 93166891
; 99453448
; 103024636
; 103543150
; 103856247
; 103914230
; 104170219
; 104253409
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0WN0U
- Formula
- C18H19Cl2NO4
- Canonical SMILES
- CCOC(=O)C1=C(NC(=C(C1C2=C(C(=CC=C2)Cl)Cl)C(=O)OC)C)C
- InChI
- 1S/C18H19Cl2NO4/c1-5-25-18(23)14-10(3)21-9(2)13(17(22)24-4)15(14)11-7-6-8-12(19)16(11)20/h6-8,15,21H,5H2,1-4H3
- InChIKey
- RZTAMFZIAATZDJ-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


