| References |
| 1 |
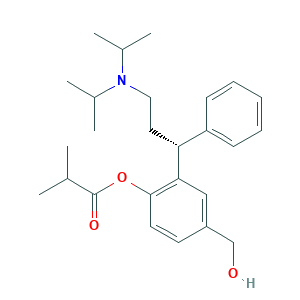
Fesoterodine was approved by FDA. The 2020 official website of the U.S. Food and Drug Administration.
|
| 2 |
Effects of the moderate CYP3A4 inhibitor, fluconazole, on the pharmacokinetics of fesoterodine in healthy subjects. Br J Clin Pharmacol. 2011 Aug;72(2):263-9.
|
| 3 |
Physiologically Based Pharmacokinetic Modeling Suggests Limited Drug-Drug Interaction for Fesoterodine When Coadministered With Mirabegron J Clin Pharmacol. 2019 Nov;59(11):1505-1518. doi: 10.1002/jcph.1438.
|
| 4 |
Population Pharmacokinetic and Pharmacodynamic Modeling of Fesoterodine in Pediatric Patients?with Neurogenic Detrusor Overactivity. Eur J Drug Metab Pharmacokinet. 2023 May;48(3):257-269. doi: 10.1007/s13318-023-00818-8.
|
| 5 |
Fesoterodine treatment of pediatric patients with neurogenic detrusor overactivity: A 24-week, randomized, open-label, phase 3 study. J Pediatr Urol. 2023 Apr;19(2):175.e1-175.e10. doi: 10.1016/j.jpurol.2022.11.020.
|
| 6 |
LABEL:FESOTERODINE FUMARATE tablet, extended release
|