| Cross-matching ID |
- PubChem CID
- 32778
- PubChem SID
-
8172825
; 15478163
; 29215430
; 34674787
; 46508780
; 48416056
; 49987582
; 51091457
; 57311407
; 75725261
; 104314493
; 127346563
; 127346564
; 134338201
; 134997083
; 136074034
; 137006664
; 139955826
; 144205252
; 160708863
; 160964607
; 164841971
; 176246077
; 179225782
; 198959702
; 215784432
; 223532541
; 226432748
; 242326209
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D04NTJ
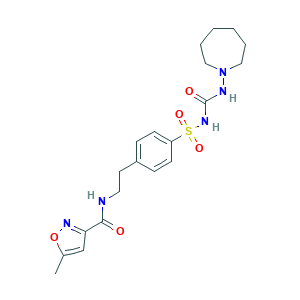
- Formula
- C20H27N5O5S
- Canonical SMILES
- CC1=CC(=NO1)C(=O)NCCC2=CC=C(C=C2)S(=O)(=O)NC(=O)NN3CCCCCC3
- InChI
- 1S/C20H27N5O5S/c1-15-14-18(23-30-15)19(26)21-11-10-16-6-8-17(9-7-16)31(28,29)24-20(27)22-25-12-4-2-3-5-13-25/h6-9,14H,2-5,10-13H2,1H3,(H,21,26)(H2,22,24,27)
- InChIKey
- ZKUDBRCEOBOWLF-UHFFFAOYSA-N
|