| Cross-matching ID |
- PubChem CID
- 14982
- PubChem SID
-
595979
; 8145710
; 8161234
; 12014953
; 14767124
; 14889369
; 29282971
; 49974013
; 53789412
; 57328816
; 80458755
; 87570348
; 93165815
; 104069368
; 104225159
; 104253562
; 104335876
; 117542241
; 118046163
; 119525684
; 124360077
; 124757704
; 125164508
; 126592383
; 134981322
; 135985736
; 137005264
; 137174069
; 144205073
; 144206976
; 144213802
; 152032505
; 152108764
; 160703999
; 162037865
; 162176852
; 163614442
; 163883554
; 175265976
; 178101402
; 179316054
; 184545284
; 196378207
; 223673136
; 223721543
; 223800488
; 226407764
; 249839445
; 252216692
; 252391202
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0T8QB
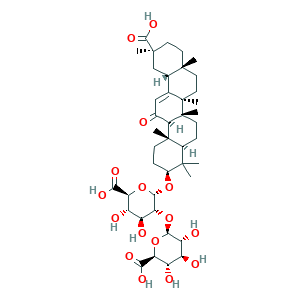
- Formula
- C42H62O16
- Canonical SMILES
- CC1(C2CCC3(C(C2(CCC1OC4C(C(C(C(O4)C(=O)O)O)O)OC5C(C(C(C(O5)C(=O)O)O)O)O)C)C(=O)C=C6C3(CCC7(C6CC(CC7)(C)C(=O)O)C)C)C)C
- InChI
- 1S/C42H62O16/c1-37(2)21-8-11-42(7)31(20(43)16-18-19-17-39(4,36(53)54)13-12-38(19,3)14-15-41(18,42)6)40(21,5)10-9-22(37)55-35-30(26(47)25(46)29(57-35)33(51)52)58-34-27(48)23(44)24(45)28(56-34)32(49)50/h16,19,21-31,34-35,44-48H,8-15,17H2,1-7H3,(H,49,50)(H,51,52)(H,53,54)/t19-,21-,22-,23-,24-,25-,26-,27+,28-,29-,30+,31+,34-,35-,38+,39-,40-,41+,42+/m0/s1
- InChIKey
- LPLVUJXQOOQHMX-QWBHMCJMSA-N
|