Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0806) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Heparin
|
|||||
| Synonyms |
Heparin [BAN]; Heparina; Heparine; Heparinum; Hepathrom; Lipo-hepin; Liquaemin; Liquemin; Lovenox; Lovenox HP; Low molecular weight heparin; Multiparin; Nadroparin; Nadroparine; Normiflo; Novoheparin; Octaparin; PK-10169; Pabyrin; Parnaparin; Adomiparin; Ardeparin; Bemiparin; Clexane; Clivarin; Depo-Heparin; Eparina [DCIT]; Fluxum; Fragmin A; Fragmin B; Hed-heparin; Parvoparin; Pularin; Reviparin; SEMULOPARIN; Sandoparin; Subeparin; Sublingula; Tinzaparin; Triofiban; Vetren; Vitrum AB; enoxaparin; heparin; 9005-49-6; CY 216; FR 860; LMWH
|
|||||
| Indication | Angina pectoris [ICD11: BA40] | Approved | [1] | |||
| Structure |
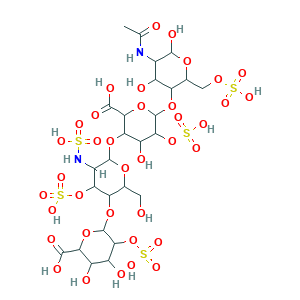 |
|||||
| 3D MOL is unavailable | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 1134.9 | Topological Polar Surface Area | 652 | ||
| Heavy Atom Count | 70 | Rotatable Bond Count | 21 | |||
| Hydrogen Bond Donor Count | 15 | Hydrogen Bond Acceptor Count | 38 | |||
| Cross-matching ID |
|
|||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

