| Synonyms |
Idamycin; Idarubicin Hcl; Idarubicin [INN:BAN]; Idarubicina; Idarubicina [INN-Spanish]; Idarubicine; Idarubicine [INN-French]; Idarubicinum; Idarubicinum [INN-Latin]; ZRP63D75JW; Zavedos; idarubicin hydrochloride; 4-Demethoxydaunomycin; Daunomycin, 4-demethoxy-; IDARUBICIN; IMI 30; IMI-30; 4-Demethoxydaunorubicin; 5,12-Naphthacenedione, 9-acetyl-7-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,9,11-trihydroxy-, (7S-cis)-; 58957-92-9; CCRIS 5083; CHEBI:42068; NSC-256439; UNII-ZRP63D75JW
|
| Cross-matching ID |
- PubChem CID
- 42890
- PubChem SID
-
794734
; 7887077
; 7979585
; 8177897
; 11378086
; 14810780
; 14908709
; 17405162
; 24278488
; 26697284
; 26704230
; 26704281
; 34708383
; 46506973
; 47959947
; 48416101
; 49995025
; 50106402
; 50106403
; 50106404
; 53777706
; 53788391
; 57312753
; 79820214
; 85789484
; 87325144
; 90340569
; 92303774
; 92308741
; 96024754
; 103311042
; 104340903
; 117597729
; 117695696
; 121361286
; 124749845
; 124891657
; 126626724
; 126661729
; 127301008
; 127301009
; 127301010
; 127301011
; 127301012
; 127301013
; 127301014
; 127301015
; 127301016
; 127301017
; 127301018
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D01XDL
- Formula
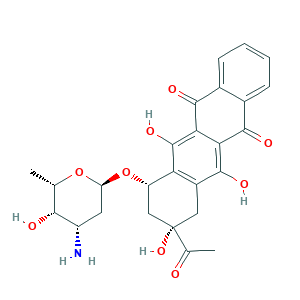
- C26H27NO9
- Canonical SMILES
- CC1C(C(CC(O1)OC2CC(CC3=C2C(=C4C(=C3O)C(=O)C5=CC=CC=C5C4=O)O)(C(=O)C)O)N)O
- InChI
- 1S/C26H27NO9/c1-10-21(29)15(27)7-17(35-10)36-16-9-26(34,11(2)28)8-14-18(16)25(33)20-19(24(14)32)22(30)12-5-3-4-6-13(12)23(20)31/h3-6,10,15-17,21,29,32-34H,7-9,27H2,1-2H3/t10-,15-,16-,17-,21+,26-/m0/s1
- InChIKey
- XDXDZDZNSLXDNA-TZNDIEGXSA-N
|