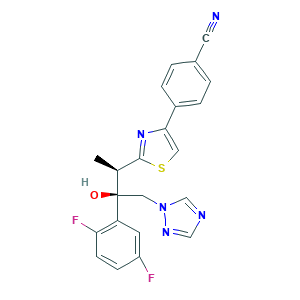
| Synonyms |
Isavuconazole; Isavuconazole (INN); Isavuconazole [INN]; Isavuconazole(BAL-4815; RO 0094815; RO-0094815; RO-0094815); BAL-8557; BAL 4815; BAL-4815; isavuconazol; 241479-67-4; 4-[2-[(1R,2R)-2-(2,5-Difluorophenyl)-2-hydroxy-1-methyl-3-(1H-1,2,4-triazol-1-yl)propyl]-4-thiazolyl]benzonitrile; 4-[2-[(2R,3R)-3-(2,5-difluorophenyl)-3-hydroxy-4-(1,2,4-triazol-1-yl)butan-2-yl]-1,3-thiazol-4-yl]benzonitrile; 60UTO373KE; CHEBI:85979; UNII-60UTO373KE
|
| Cross-matching ID |
- PubChem CID
- 6918485
- PubChem SID
-
12015361
; 14856922
; 17194920
; 24722773
; 43529855
; 57371952
; 78488413
; 103570494
; 114788046
; 126680912
; 135261044
; 137006498
; 142496862
; 164227664
; 164761259
; 172918182
; 198957657
; 223665458
; 225093825
; 227200670
; 247264052
; 252150286
; 252215608
; 252479215
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D07VHS
- Formula
- C22H17F2N5OS
- Canonical SMILES
- CC(C1=NC(=CS1)C2=CC=C(C=C2)C#N)C(CN3C=NC=N3)(C4=C(C=CC(=C4)F)F)O
- InChI
- 1S/C22H17F2N5OS/c1-14(21-28-20(10-31-21)16-4-2-15(9-25)3-5-16)22(30,11-29-13-26-12-27-29)18-8-17(23)6-7-19(18)24/h2-8,10,12-14,30H,11H2,1H3/t14-,22+/m0/s1
- InChIKey
- DDFOUSQFMYRUQK-RCDICMHDSA-N
|