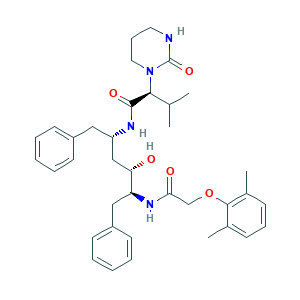
| Synonyms |
Lopinavir; Lopinavir (ABT-378); RS-346; Aluviran; Kaletra; Koletra; (alphaS)-Tetrahydro-N-((alphaS)-alpha-((2S,3S)-2-hydroxy-4-phenyl-3-(2-(2,6-xylyloxy)acetamido)butyl)phenethyl)-alpha-isopropyl-2-oxo-1(2H)-pyrimidineacetamide; 192725-17-0; 2494G1JF75; A 157378; A 157378.0; A-157378-0; A-157378.0; ABT 378; ABT-378; CHEBI:31781; DSSTox_RID_81630; LPV; N-{1-BENZYL-4-[2-(2,6-DIMETHYL-PHENOXY)-ACETYLAMINO]-3-HYDROXY-5-PHENYL-PENTYL}-3-METHYL-2-(2-OXO-TETRAHYDRO-PYRIMIDIN-1-YL)-BUTYRAMIDE; NCGC00164576-02; UNII-2494G1JF75
|
| Cross-matching ID |
- PubChem CID
- 92727
- PubChem SID
-
583260
; 615079
; 826916
; 841952
; 7848488
; 7885634
; 7979791
; 10225535
; 11108093
; 11528784
; 14912304
; 14912305
; 26737285
; 26757998
; 44423527
; 46392556
; 46508588
; 49853992
; 50086984
; 50096471
; 50096475
; 50111692
; 53812811
; 53812974
; 56310583
; 57335304
; 76999753
; 87557375
; 87557376
; 99437252
; 103198077
; 104178992
; 104408196
; 123080504
; 124757206
; 124893167
; 125164010
; 126522368
; 126655919
; 126665841
; 127310211
; 127310212
; 127310213
; 127338643
; 127338644
; 127338645
; 134337997
; 135051036
; 136283935
; 136367896
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0U5GB
- Formula
- C37H48N4O5
- Canonical SMILES
- CC1=C(C(=CC=C1)C)OCC(=O)NC(CC2=CC=CC=C2)C(CC(CC3=CC=CC=C3)NC(=O)C(C(C)C)N4CCCNC4=O)O
- InChI
- 1S/C37H48N4O5/c1-25(2)34(41-20-12-19-38-37(41)45)36(44)39-30(21-28-15-7-5-8-16-28)23-32(42)31(22-29-17-9-6-10-18-29)40-33(43)24-46-35-26(3)13-11-14-27(35)4/h5-11,13-18,25,30-32,34,42H,12,19-24H2,1-4H3,(H,38,45)(H,39,44)(H,40,43)/t30-,31-,32-,34-/m0/s1
- InChIKey
- KJHKTHWMRKYKJE-SUGCFTRWSA-N
|