| General Information of Drug (ID:
DR0978) |
| Drug Name |
Lorazepam
|
| Synonyms |
Larpose; Laubeel; Lorabenz; Lorapam; Lorazene; Lorazep; Lorazepam Intensol; Lorazin; Lorenin; Loridem; Lorsedal; Lozepam; Merlit; Norlormetazepam; Novhepar; Orfidal; Punktyl; Renaquil; Rocosgen; Sedatival; Sedizepan; Sidenar; Silence; Sinestron; Almazine; Anxiedin; Anzepam; Aplacasse; Aplacassee; Apo-Lorazepam; Ativan; Azurogen; Bonatranquan; Delormetazepam; Duralozam; Efasedan; Emotival; Equitam; Idalprem; Kalmalin; Stapam; Temesta; Tranqipam; Trapax; lorazepam; o-Chlorooxazepam; o-Chloroxazepam; 846-49-1; Donix; Lorax; Loraz; Quait; Tavor; Tolid; Wypax
|
| Indication |
Anxiety disorder
[ICD11: 6B00]
|
Approved
|
[1]
|
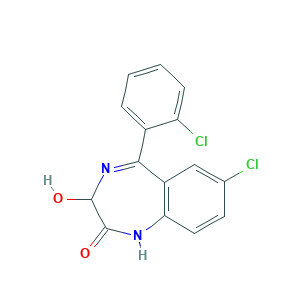
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
321.2 |
Topological Polar Surface Area |
61.7 |
| Heavy Atom Count |
21 |
Rotatable Bond Count |
1 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
3 |
| Cross-matching ID |
- PubChem CID
- 3958
- PubChem SID
-
145006
; 597377
; 3249123
; 4964189
; 7847431
; 7849642
; 7979801
; 8150120
; 8152485
; 10536882
; 11336140
; 11361379
; 11462351
; 14801418
; 24896291
; 29215381
; 29223072
; 46386878
; 46508468
; 48035217
; 48416184
; 49884087
; 50340424
; 53789194
; 56313651
; 57322068
; 92308613
; 99233258
; 99302053
; 103179427
; 104134178
; 104305018
; 124659146
; 124759848
; 125536697
; 126674761
; 127741279
; 131327750
; 134337551
; 134979720
; 135697881
; 137008105
; 137201885
; 140053026
; 144097671
; 144205222
; 152043722
; 160963534
; 162022367
; 162725399
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0E0OG
- Formula
- C15H10Cl2N2O2
- Canonical SMILES
- C1=CC=C(C(=C1)C2=NC(C(=O)NC3=C2C=C(C=C3)Cl)O)Cl
- InChI
- 1S/C15H10Cl2N2O2/c16-8-5-6-12-10(7-8)13(19-15(21)14(20)18-12)9-3-1-2-4-11(9)17/h1-7,15,21H,(H,18,20)
- InChIKey
- DIWRORZWFLOCLC-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


