| PDM Name |
PDM ID |
PubChem ID |
Reaction |
PDM Level |
Biosystem |
|
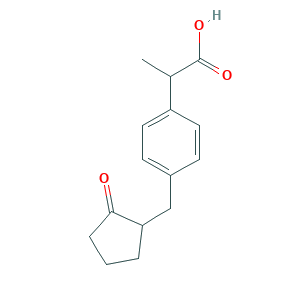
CS-600G M1
|
PDM007805
|
|
Oxidation
-
alpha-Hydroxylation of carbonyl group |
1 |
Human
|
|
CS-600G M1
|
PDM007805
|
|
Oxidation
-
Hydroxylation of non-terminal aliphatic carbon adjacent to aromatic ring |
1 |
Human
|
|
CS-600G M10
|
PDM007813
|
|
Reduction
-
Reduction of ketone to alcohol |
1 |
Human
|
|
CS-600G M2
|
PDM007806
|
N. A. |
Oxidation
-
Hydroxylation of non-terminal aliphatic carbon adjacent to aromatic ring |
1 |
Human
|
|
CS-600G M3
|
PDM007807
|
N. A. |
Oxidation
-
Hydroxylation of terminal methyl |
1 |
Human
|
|
CS-600G M4
|
PDM007808
|
N. A. |
Other reaction
-
Terminal desaturation |
1 |
Human
|
|
CS-600G M43
|
PDM007846
|
N. A. |
Conjugation
-
Glycine conjugation |
1 |
Human
|
|
CS-600G M44
|
PDM007847
|
N. A. |
Conjugation
-
O-Glucuronidation of aliphatic acid |
1 |
Human
|
|
CS-600G M45
|
PDM007848
|
N. A. |
Conjugation
-
Carnitine conjugation |
1 |
Human
|
|
CS-600G M6
|
PDM007809
|
|
Oxidation
-
alpha-Hydroxylation of carbonyl group |
1 |
Human
|
|
CS-600G M7
|
PDM007810
|
N. A. |
Oxidation
-
alpha-Hydroxylation of carbonyl group |
1 |
Human
|
|
CS-600G M8
|
PDM007811
|
N. A. |
Oxidation
-
Hydroxylation of alicyclic secondary carbon |
1 |
Human
|
|
CS-600G M9
|
PDM007812
|
N. A. |
Oxidation
-
Hydroxylation of alicyclic secondary carbon |
1 |
Human
|
|
CS-600G M11
|
PDM007814
|
N. A. |
Conjugation
-
Alkyl-OH-glucuronidation |
2 |
Human
|
|
CS-600G M12
|
PDM007815
|
N. A. |
Conjugation
-
O-Glucuronidation of aliphatic acid |
2 |
Human
|
|
CS-600G M13
|
PDM007816
|
N. A. |
Conjugation
-
Glycine conjugation |
2 |
Human
|
|
CS-600G M14
|
PDM007817
|
N. A. |
Conjugation
-
Alkyl-OH-glucuronidation |
2 |
Human
|
|
CS-600G M15
|
PDM007818
|
N. A. |
Conjugation
-
O-Glucuronidation of aliphatic acid |
2 |
Human
|
|
CS-600G M16
|
PDM007819
|
N. A. |
Conjugation
-
Sulfation of secondary alcohol |
2 |
Human
|
|
CS-600G M17
|
PDM007820
|
N. A. |
Conjugation
-
Glycine conjugation |
2 |
Human
|
|
CS-600G M18
|
PDM007821
|
N. A. |
Conjugation
-
Alkyl-OH-glucuronidation |
2 |
Human
|
|
CS-600G M19
|
PDM007822
|
N. A. |
Conjugation
-
O-Glucuronidation of aliphatic acid |
2 |
Human
|
|
CS-600G M20
|
PDM007823
|
N. A. |
Conjugation
-
Sulfation of primary alcohol |
2 |
Human
|
|
CS-600G M21
|
PDM007824
|
N. A. |
Conjugation
-
Glycine conjugation |
2 |
Human
|
|
CS-600G M22
|
PDM007825
|
N. A. |
Conjugation
-
O-Glucuronidation of aliphatic acid |
2 |
Human
|
|
CS-600G M23
|
PDM007826
|
N. A. |
Conjugation
-
Glycine conjugation |
2 |
Human
|
|
CS-600G M24
|
PDM007827
|
N. A. |
Conjugation
-
Alkyl-OH-glucuronidation |
2 |
Human
|
|
CS-600G M25
|
PDM007828
|
N. A. |
Conjugation
-
O-Glucuronidation of aliphatic acid |
2 |
Human
|
|
CS-600G M26
|
PDM007829
|
N. A. |
Conjugation
-
Glycine conjugation |
2 |
Human
|
|
CS-600G M27
|
PDM007830
|
N. A. |
Conjugation
-
Alkyl-OH-glucuronidation |
2 |
Human
|
|
CS-600G M28
|
PDM007831
|
N. A. |
Conjugation
-
O-Glucuronidation of aliphatic acid |
2 |
Human
|
|
CS-600G M29
|
PDM007832
|
N. A. |
Conjugation
-
Sulfation of secondary alcohol |
2 |
Human
|
|
CS-600G M30
|
PDM007833
|
N. A. |
Conjugation
-
Glycine conjugation |
2 |
Human
|
|
CS-600G M31
|
PDM007834
|
N. A. |
Conjugation
-
Alkyl-OH-glucuronidation |
2 |
Human
|
|
CS-600G M32
|
PDM007835
|
N. A. |
Conjugation
-
O-Glucuronidation of aliphatic acid |
2 |
Human
|
|
CS-600G M33
|
PDM007836
|
N. A. |
Conjugation
-
Sulfation of secondary alcohol |
2 |
Human
|
|
CS-600G M34
|
PDM007837
|
N. A. |
Conjugation
-
Glycine conjugation |
2 |
Human
|
|
CS-600G M35
|
PDM007838
|
N. A. |
Conjugation
-
Alkyl-OH-glucuronidation |
2 |
Human
|
|
CS-600G M36
|
PDM007839
|
N. A. |
Conjugation
-
O-Glucuronidation of aliphatic acid |
2 |
Human
|
|
CS-600G M37
|
PDM007840
|
N. A. |
Conjugation
-
Sulfation of secondary alcohol |
2 |
Human
|
|
CS-600G M38
|
PDM007841
|
N. A. |
Conjugation
-
Glycine conjugation |
2 |
Human
|
|
CS-600G M39
|
PDM007842
|
N. A. |
Conjugation
-
Alkyl-OH-glucuronidation |
2 |
Human
|
|
CS-600G M40
|
PDM007843
|
N. A. |
Conjugation
-
O-Glucuronidation of aliphatic acid |
2 |
Human
|
|
CS-600G M41
|
PDM007844
|
N. A. |
Conjugation
-
Sulfation of secondary alcohol |
2 |
Human
|
|
CS-600G M42
|
PDM007845
|
|
Conjugation
-
Glycine conjugation |
2 |
Human
|
|
|
|
|
|
|