| General Information of Drug (ID:
DR1128) |
| Drug Name |
Naltrexone
|
| Synonyms |
Naltrexona; Naltrexona [INN-Spanish]; Naltrexone [USAN:INN:BAN]; Naltrexone hydrochloride; Naltrexonum; Naltrexonum [INN-Latin]; Celupan; PTI-555; UM-792; Vivitrex; Vivitrol; naltrexone; (-)-Naltrexone; 16590-41-3; 17-(Cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxymorphinan-6-one; 17-(Cyclopropylmethyl)-4,5alpha-epoxy-3,14-dihydroxymorphinan-6-one; 5S6W795CQM; BRN 3596648; CCRIS 3506; CHEBI:7465; EINECS 240-649-9; HSDB 6750; N-Cyclopropylmethyl-14-hydroxydihydromorphinone; N-Cyclopropylmethylnoroxymorphone; ReVia; UNII-5S6W795CQM
|
| Indication |
Alcohol dependence
[ICD11: 6C40]
|
Approved
|
[1]
|
| Structure |
|
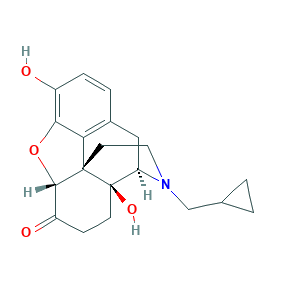 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
341.4 |
Topological Polar Surface Area |
70 |
| Heavy Atom Count |
25 |
Rotatable Bond Count |
2 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
5 |
| Cross-matching ID |
- PubChem CID
- 5360515
- PubChem SID
-
9462
; 596067
; 11466144
; 11467264
; 11485807
; 11653758
; 14753731
; 14875729
; 39383995
; 46505333
; 47206838
; 47350797
; 47647883
; 47722000
; 48095907
; 48170809
; 48244798
; 49698353
; 49993104
; 53787920
; 57362014
; 80591843
; 85787880
; 92308967
; 93166563
; 93815010
; 103915329
; 104222111
; 124886894
; 124886895
; 127334962
; 127334963
; 127334964
; 127334965
; 127334966
; 127770775
; 131283791
; 134337689
; 134993601
; 135650687
; 137001356
; 141566780
; 143492741
; 144205560
; 152134015
; 152233304
; 152258923
; 160647768
; 160964048
; 166653673
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0PG8O
- Formula
- C20H23NO4
- Canonical SMILES
- C1CC1CN2CCC34C5C(=O)CCC3(C2CC6=C4C(=C(C=C6)O)O5)O
- InChI
- 1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1
- InChIKey
- DQCKKXVULJGBQN-XFWGSAIBSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


