| Synonyms |
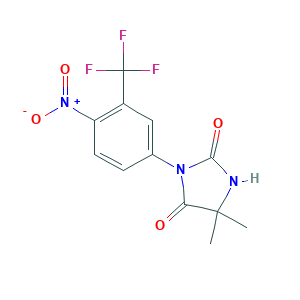
Nilandron; Nilandrone; Nilutamida; Nilutamida [Spanish]; Nilutamide [USAN:INN:BAN]; Nilutamidum; Nilutamidum [Latin]; RU 23908; Anandron; RU 23908-10; RU-23908; XWXYUMMDTVBTOU-UHFFFAOYSA-N; nilutamide; 5,5-Dimethyl-3-(alpha,alpha,alpha-trifluoro-4-nitro-m-tolyl)hydantoin; 5,5-dimethyl-3-(4-nitro-3-(trifluoromethyl)phenyl)imidazolidine-2,4-dione; 5,5-dimethyl-3-[4-nitro-3-(trifluoromethyl)phenyl]imidazolidine-2,4-dione; 63612-50-0; BRN 0841906; C12H10F3N3O4; CHEBI:7573; CHEMBL1274; UNII-51G6I8B902
|
| Cross-matching ID |
- PubChem CID
- 4493
- PubChem SID
-
10364
; 5851647
; 7404097
; 7848028
; 7978498
; 8152766
; 11111555
; 11111556
; 11114201
; 11342173
; 11362356
; 11364519
; 11367081
; 11369643
; 11372644
; 11374239
; 11377805
; 11466956
; 11468076
; 11485861
; 11486751
; 11487758
; 11489789
; 11491448
; 11492415
; 11495439
; 12013485
; 14850346
; 17405438
; 24278596
; 26612639
; 26680190
; 26747174
; 26747175
; 26752242
; 29223587
; 46505381
; 47365306
; 47365307
; 47959867
; 48110544
; 48259346
; 48259347
; 48334600
; 49698835
; 49980214
; 50104947
; 50104948
; 50104949
; 50104950
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0SN9T
- Formula
- C12H10F3N3O4
- Canonical SMILES
- CC1(C(=O)N(C(=O)N1)C2=CC(=C(C=C2)[N+](=O)[O-])C(F)(F)F)C
- InChI
- 1S/C12H10F3N3O4/c1-11(2)9(19)17(10(20)16-11)6-3-4-8(18(21)22)7(5-6)12(13,14)15/h3-5H,1-2H3,(H,16,20)
- InChIKey
- XWXYUMMDTVBTOU-UHFFFAOYSA-N
|