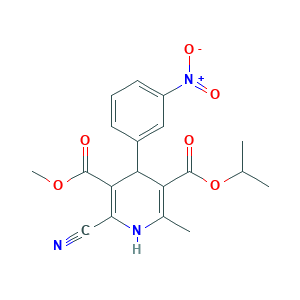
| Synonyms |
Nilvadipine (ARC029); Nilvadipine [USAN:INN:JAN]; Nilvadipino [Spanish]; Nilvadipinum [Latin]; Nivadil; Nivadipine; SK&F 102,362; SK&F-102362; nilvadipine; 3-O-methyl 5-O-propan-2-yl 2-cyano-6-methyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate; 5-Isopropyl 3-methyl 2-cyano-1,4-dihydro-6-methyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate; 75530-68-6; BRN 3572609; C19H19N3O6; CL 287,389; CL-287389; Escor; FK 235; FK-235; FR 34235; FR-34235; FR34235; ARC029; FAIIFDPAEUKBEP-UHFFFAOYSA-N; NCGC00167435-01
|