| General Information of Drug (ID:
DR1262) |
| Drug Name |
Permethrin
|
| Synonyms |
Perigen; Permasect; Permethrin; Permethrine; Permetrina; Perthrine; Picket; Pounce; Pramex; Quamlin; Stomoxi; Stomoxin; Stomozan; Transpermethrin; Acticin; Ambush; Ambushfog; Chinetrin; Coopex; Corsair; Dragnet; Ecsumin; Ectiban; Efmethrin; Eksmin; Elimite; FMC 35171; Imperator; Indothrin; Kaleait; Kestrel; Lyclear; NRDC 146; NRDC 148; Outflank; Transpermethrin [ISO]; trans-(+-)-Permethrin; (+)-trans-Permethrin; (+-)-cis-Fmc 33297; (+-)-cis-Permethrin; (+-)-trans-Permethrin; 1RS,cis-Permethrin; 1RS-trans-Permethrin; 52645-53-1; BRN 4153590; Kudos
|
| Indication |
Scabies
[ICD11: 1G04]
|
Approved
|
[1]
|
| Structure |
|
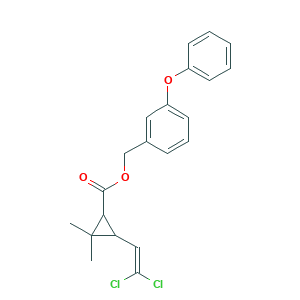 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
391.3 |
Topological Polar Surface Area |
35.5 |
| Heavy Atom Count |
26 |
Rotatable Bond Count |
7 |
| Hydrogen Bond Donor Count |
0 |
Hydrogen Bond Acceptor Count |
3 |
| Cross-matching ID |
- PubChem CID
- 40326
- PubChem SID
-
5646900
; 8176555
; 10525033
; 10525292
; 10526594
; 14854178
; 17395388
; 24868001
; 24869115
; 24869349
; 24871630
; 24899290
; 26675675
; 26754479
; 26754481
; 26754482
; 26754483
; 26754484
; 29215320
; 29215321
; 34706112
; 46505374
; 47207113
; 48415484
; 48421679
; 48425983
; 49833360
; 49959692
; 49959696
; 50109850
; 56310674
; 56312325
; 56314259
; 57260102
; 57260103
; 57288423
; 57312421
; 57650455
; 57650562
; 74717839
; 85088539
; 85189547
; 91696064
; 103559256
; 104334065
; 117452446
; 118783839
; 121362319
; 124800250
; 124892140
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0K8MP
- Formula
- C21H20Cl2O3
- Canonical SMILES
- CC1(C(C1C(=O)OCC2=CC(=CC=C2)OC3=CC=CC=C3)C=C(Cl)Cl)C
- InChI
- 1S/C21H20Cl2O3/c1-21(2)17(12-18(22)23)19(21)20(24)25-13-14-7-6-10-16(11-14)26-15-8-4-3-5-9-15/h3-12,17,19H,13H2,1-2H3
- InChIKey
- RLLPVAHGXHCWKJ-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


