| General Information of Drug (ID:
DR1285) |
| Drug Name |
Pilocarpine
|
| Synonyms |
Pilocarpin; Pilocarpine chloride; Pilocarpine monohydrochloride; Pilocarpol; Pilokarpin; Pilokarpol; Salagen; Spersacarpine; Syncarpine; beta-Pilocarpine hydrochloride; pilocarpine; (+)-Pilocarpine; (3S,4R)-3-Ethyl-4-((1-methyl-1H-imidazol-5-yl)methyl)dihydrofuran-2(3H)-one; (3S,4R)-3-ethyl-4-[(1-methyl-1H-imidazol-5-yl)methyl]dihydrofuran-2(3H)-one; 92-13-7; AI3-50523; CHEMBL550; EINECS 202-128-4; HSDB 3163; UNII-01MI4Q9DI3; Epicar; Isoptocarpine; Mi-Pilo; Ocusert P 20; Ocusert Pilo-40; Ocusert pilo; Ocusert pilo-20
|
| Indication |
Glaucoma
[ICD11: 9C61]
|
Approved
|
[1]
|
| Structure |
|
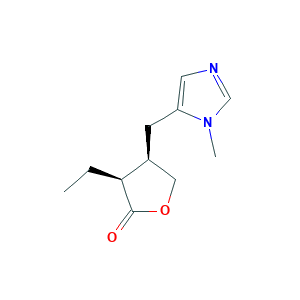 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
208.26 |
Topological Polar Surface Area |
44.1 |
| Heavy Atom Count |
15 |
Rotatable Bond Count |
3 |
| Hydrogen Bond Donor Count |
0 |
Hydrogen Bond Acceptor Count |
3 |
| Cross-matching ID |
- PubChem CID
- 5910
- PubChem SID
-
9677
; 605273
; 7847591
; 7980323
; 8153662
; 11113352
; 11335533
; 11360772
; 11364001
; 11366563
; 11369125
; 11371791
; 11374095
; 11377287
; 11408793
; 11461744
; 11466477
; 11467597
; 11485023
; 11486173
; 11489100
; 11490362
; 11492293
; 11494921
; 11537578
; 15195683
; 15195684
; 25631341
; 26697419
; 26751548
; 29204486
; 29224937
; 46507475
; 47213197
; 47291056
; 47291057
; 47365110
; 47440175
; 47588917
; 47736396
; 47885334
; 48110373
; 48259150
; 48414341
; 48416432
; 49698499
; 49857637
; 50070744
; 50104139
; 50104140
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D06HLY
- Formula
- C11H16N2O2
- Canonical SMILES
- CCC1C(COC1=O)CC2=CN=CN2C
- InChI
- 1S/C11H16N2O2/c1-3-10-8(6-15-11(10)14)4-9-5-12-7-13(9)2/h5,7-8,10H,3-4,6H2,1-2H3/t8-,10-/m0/s1
- InChIKey
- QCHFTSOMWOSFHM-WPRPVWTQSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


