| General Information of Drug (ID:
DR1510) |
| Drug Name |
J-005528
|
| Synonyms |
Alphazole; Amidoxal; Chemouag; Sulfafurazole; Cosoxazole; Gantrisin; Gantrisona; Gantrosan; Isoxamin; Neazolin; Neoxazol; Pancid; Renosulfan; Roxosul tablets; Soxomide; Sulfadimethylisoxazole; Sulfafurazol; Sulfagan; Sulfaisoxazole; Sulfalar; Sulfasol; Sulfasoxazole; Sulfazin; Sulfisonazole; Sulfisoxasole; Sulfisoxazol; Sulfisoxazole dialamine; Sulfizin; Sulfofurazole; Sulfoxol; Sulphafurazol; Sulphafurazole; Sulphafurazolum; Sulphaisoxazole; Sulphisoxazol; Sulphofurazole; Sulsoxin; Unisulf; Uritrisin; sulfisoxazole; 127-69-5; Accuzole; Sosol
|
| Indication |
Infectious cystitis
[ICD11: GC00]
|
Phase 2
|
[1]
|
| Structure |
|
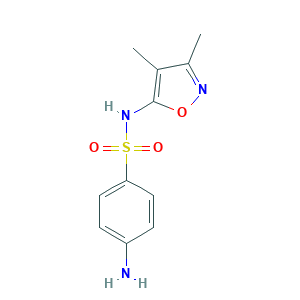 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
267.31 |
Topological Polar Surface Area |
107 |
| Heavy Atom Count |
18 |
Rotatable Bond Count |
3 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
6 |
| Cross-matching ID |
- PubChem CID
- 5344
- PubChem SID
-
9526
; 77649
; 91794
; 94502
; 519811
; 603111
; 855841
; 859862
; 5632698
; 7847516
; 7980707
; 8001163
; 8149542
; 8153283
; 10321680
; 10506243
; 10589442
; 11112277
; 11113344
; 11335754
; 11360993
; 11363918
; 11366480
; 11369042
; 11372695
; 11373896
; 11377204
; 11461965
; 11466362
; 11467482
; 11485185
; 11486024
; 11489141
; 11491543
; 11492164
; 11494838
; 14848258
; 17389726
; 17390719
; 24707909
; 24899697
; 26611934
; 26679889
; 26747009
; 26751527
; 29224396
; 46505342
; 47291108
; 47440228
; 47662260
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D09TBD
- Formula
- C11H13N3O3S
- Canonical SMILES
- CC1=C(ON=C1C)NS(=O)(=O)C2=CC=C(C=C2)N
- InChI
- 1S/C11H13N3O3S/c1-7-8(2)13-17-11(7)14-18(15,16)10-5-3-9(12)4-6-10/h3-6,14H,12H2,1-2H3
- InChIKey
- NHUHCSRWZMLRLA-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


