| Synonyms |
Tenapanor; Tenapanor [USAN:INN]; WYD79216A6; 1234423-95-0; 3-((S)-6,8-dichloro-2-methyl-1,2,3,4-tetrahydroisoquinolin-4-yl)-N-(26-((3-((S)-6,8-dichloro-2-methyl-1,2,3,4-tetrahydroisoquinolin-4-yl)phenyl)sulfonamido)-10,17-dioxo-3,6,21,24-tetraoxa-9,11,16,18-tetraazahexacosyl)benzenesulfonamide; Ibsrela; RDX 5791; RDX-5791; RDX5791; AZD 1722; AZD-1722; AZD1722; CHEMBL3304485; DTXSID40154016; EX-A2506; GTPL8449; SCHEMBL15267600; UNII-WYD79216A6
|
| Cross-matching ID |
- PubChem CID
- 71587953
- PubChem SID
-
163643268
; 198966338
; 240221763
; 242654674
; 252166659
- CAS Number
-
- TTD Drug ID
- D04TQO
- Formula
- C50H66Cl4N8O10S2
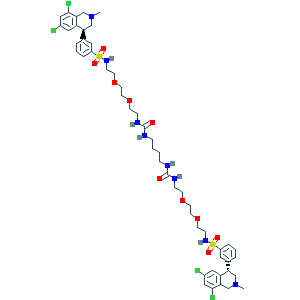
- Canonical SMILES
- CN1CC(C2=C(C1)C(=CC(=C2)Cl)Cl)C3=CC(=CC=C3)S(=O)(=O)NCCOCCOCCNC(=O)NCCCCNC(=O)NCCOCCOCCNS(=O)(=O)C4=CC=CC(=C4)C5CN(CC6=C5C=C(C=C6Cl)Cl)C
- InChI
- 1S/C50H66Cl4N8O10S2/c1-61-31-43(41-27-37(51)29-47(53)45(41)33-61)35-7-5-9-39(25-35)73(65,66)59-15-19-71-23-21-69-17-13-57-49(63)55-11-3-4-12-56-50(64)58-14-18-70-22-24-72-20-16-60-74(67,68)40-10-6-8-36(26-40)44-32-62(2)34-46-42(44)28-38(52)30-48(46)54/h5-10,25-30,43-44,59-60H,3-4,11-24,31-34H2,1-2H3,(H2,55,57,63)(H2,56,58,64)/t43-,44-/m0/s1
- InChIKey
- DNHPDWGIXIMXSA-CXNSMIOJSA-N
|