| General Information of Drug (ID:
DR1552) |
| Drug Name |
Tenofovir alafenamide
|
| Prodrug Info |
Tenofovir alafenamide is the prodrug of Tenofovir(TFV)
|
| Synonyms |
Tenofovir Alafenamide (GS-7340); Tenofovir Alafenamide [USAN:INN]; UNII-EL9943AG5J; UNII-J4414G3BUK; Vemlidy; Tenofovir; UNII-W4HFE001U5; W4HFE001U5; (R)-(((1-(6-AMINO-9H-PURIN-9-YL)PROPAN-2-YL)OXY)METHYL)PHOSPHONIC ACID; (R)-(1-(6-amino-9H-purin-9-yl)propan-2-yloxy)methylphosphonic acid; (R)-9-(2-Phosphonomethoxypropyl)adenine; (R)-9-[2-(Phosphonomethoxy)propyl]adenine; (R)-PMPA; ({[(2R)-1-(6-amino-9H-purin-9-yl)propan-2-yl]oxy}methyl)phosphonic acid; 147127-20-6; 9-[(R)-2-(phosphonomethoxy)propyl]adenine; Apropovir; CHEBI:63625; D,L-Tenofovir; GS-1278; PMPA; PMPA gel; (S)-Isopropyl 2-(((S)-((((R)-1-(6-amino-9H-purin-9-yl)propan-2-yl)oxy)methyl)(phenoxy)phosphoryl)amino)propanoate; 379270-37-8; AK109983; EL9943AG5J; GS 7340; GS-7339; GS-7340; GS7340; J4414G3BUK; L-Alanine, N-((S)-(((1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy)methyl)phenoxyphosphinyl)-, 1-methylethyl ester; TENOFOVIR ALAFENAMIDE
|
| Indication |
Viral hepatitis
[ICD11: 1E51]
|
Approved
|
[1]
|
| Structure |
|
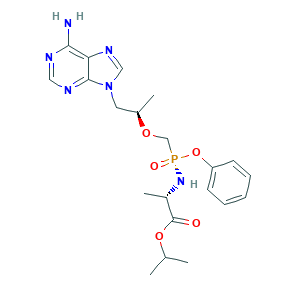 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
476.5 |
Topological Polar Surface Area |
144 |
| Heavy Atom Count |
33 |
Rotatable Bond Count |
12 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
10 |
| Cross-matching ID |
- PubChem CID
- 9574768
- ChEBI ID
-
- CAS Number
-
- Formula
- C21H29N6O5P
- Canonical SMILES
- CC(C)OC(=O)C(C)NP(=O)(COC(C)CN1C=NC2=C(N=CN=C21)N)OC3=CC=CC=C3
- InChI
- 1S/C21H29N6O5P/c1-14(2)31-21(28)16(4)26-33(29,32-17-8-6-5-7-9-17)13-30-15(3)10-27-12-25-18-19(22)23-11-24-20(18)27/h5-9,11-12,14-16H,10,13H2,1-4H3,(H,26,29)(H2,22,23,24)/t15-,16+,33+/m1/s1
- InChIKey
- LDEKQSIMHVQZJK-CAQYMETFSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


