| Cross-matching ID |
- PubChem CID
- 54675776
- PubChem SID
-
8799
; 597088
; 855950
; 7847269
; 7980765
; 8144439
; 11336002
; 11361241
; 11363928
; 11366490
; 11369052
; 11371550
; 11373922
; 11377214
; 11462213
; 11466168
; 11467288
; 11485193
; 11485895
; 11489145
; 11490381
; 11492167
; 11494848
; 14759560
; 14930699
; 24888860
; 24900120
; 25643873
; 36634859
; 39289915
; 39404343
; 43135372
; 46506693
; 47216820
; 47515360
; 47662329
; 47662330
; 47810796
; 48035170
; 48035171
; 48416604
; 49698367
; 53790074
; 53790289
; 56311360
; 56311878
; 56313073
; 56313451
; 56314320
; 57288852
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0Z5PF
- Formula
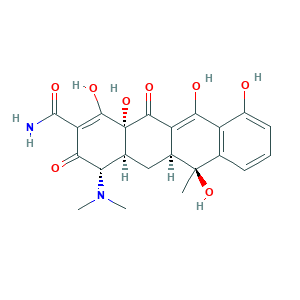
- C22H24N2O8
- Canonical SMILES
- CC1(C2CC3C(C(=O)C(=C(C3(C(=O)C2=C(C4=C1C=CC=C4O)O)O)O)C(=O)N)N(C)C)O
- InChI
- 1S/C22H24N2O8/c1-21(31)8-5-4-6-11(25)12(8)16(26)13-9(21)7-10-15(24(2)3)17(27)14(20(23)30)19(29)22(10,32)18(13)28/h4-6,9-10,15,25-26,29,31-32H,7H2,1-3H3,(H2,23,30)/t9-,10-,15-,21+,22-/m0/s1
- InChIKey
- NWXMGUDVXFXRIG-WESIUVDSSA-N
|