| Synonyms |
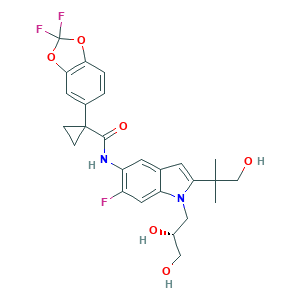
Tezacaftor; Tezacaftor [USAN]; VX 661; VX-661; (R)-1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide; 1152311-62-0; 8RW88Y506K; AK172569; C26H27F3N2O6; UNII-8RW88Y506K; VX661; cyclopropanecarboxamide, 1-(2,2-difluoro-1,3-benzodioxol-5-yl)-n-[1-[(2r)-2,3-dihydroxypropyl]-6-fluoro-2-(2-hydroxy-1,1-dimethylethyl)-1h-indol-5-yl]-
|
| Cross-matching ID |
- PubChem CID
- 46199646
- PubChem SID
-
96053596
; 120466965
; 138151262
; 138798168
; 140416866
; 163620885
; 163686208
; 163770942
; 164178299
; 165245604
; 170502668
; 184823505
; 189254077
; 224274711
; 226660026
; 235375449
; 248389873
; 249734895
; 251910604
; 252155499
; 252160789
; 252163519
; 252228808
; 252319752
; 252553650
; 252811067
- CAS Number
-
- TTD Drug ID
- D0Z5HR
- Formula
- C26H27F3N2O6
- Canonical SMILES
- CC(C)(CO)C1=CC2=CC(=C(C=C2N1CC(CO)O)F)NC(=O)C3(CC3)C4=CC5=C(C=C4)OC(O5)(F)F
- InChI
- 1S/C26H27F3N2O6/c1-24(2,13-33)22-8-14-7-18(17(27)10-19(14)31(22)11-16(34)12-32)30-23(35)25(5-6-25)15-3-4-20-21(9-15)37-26(28,29)36-20/h3-4,7-10,16,32-34H,5-6,11-13H2,1-2H3,(H,30,35)/t16-/m1/s1
- InChIKey
- MJUVRTYWUMPBTR-MRXNPFEDSA-N
|