| General Information of Drug (ID:
DR1578) |
| Drug Name |
Thiabendazole
|
| Synonyms |
Thiaben; Thiabendazol; Thiabenzole; Thibendole; Thibenzol; Thibenzole; Thibenzole 200; Thibenzole att; Tiabenda; Tiabendazol; Tiabendazole; Top Form Wormer; Triasox; thiabendazole; APL-luster; Bioguard; Bovizole; Cropasal; Eprofil; Equizole; Hokustar HP; Lombristop; Mertec; Mertect; Mertect 160; Mintesol; Mintezol; Minzolum; Mycozol; Nemapan; Omnizole; Ormogal; Pitrizet; Polival; Sistesan; Storite; Tebuzate; Tecto 10P; Tecto 40F; Tecto 60; Tecto rph; 148-79-8; 2-(4-Thiazolyl)benzimidazole; 4-(1H-Benzo[d]imidazol-2-yl)thiazole; Tbdz; Testo; Tobaz
|
| Indication |
Aspergillosis
[ICD11: 1F20]
|
Approved
|
[1]
|
| Structure |
|
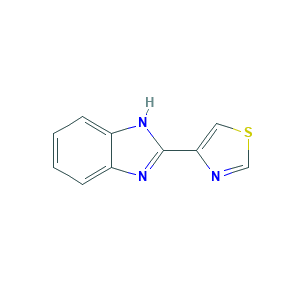 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
201.25 |
Topological Polar Surface Area |
69.8 |
| Heavy Atom Count |
14 |
Rotatable Bond Count |
1 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
3 |
| Cross-matching ID |
- PubChem CID
- 5430
- PubChem SID
-
9341
; 397296
; 481912
; 603021
; 855607
; 906414
; 3319048
; 4262837
; 5643850
; 7847438
; 7868816
; 7890801
; 7980772
; 8149550
; 8153342
; 10321759
; 10508572
; 10589481
; 11112304
; 11335247
; 11360486
; 11362813
; 11365375
; 11367937
; 11372373
; 11373926
; 11376099
; 11408671
; 11446358
; 11453179
; 11461458
; 11466552
; 11467672
; 11485217
; 11486164
; 11489147
; 11491222
; 11492169
; 11493853
; 11534461
; 14717006
; 15195537
; 17389714
; 24869246
; 24899102
; 24900297
; 24900571
; 26611942
; 26679510
; 26697097
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D08QCJ
- Formula
- C10H7N3S
- Canonical SMILES
- C1=CC=C2C(=C1)NC(=N2)C3=CSC=N3
- InChI
- 1S/C10H7N3S/c1-2-4-8-7(3-1)12-10(13-8)9-5-14-6-11-9/h1-6H,(H,12,13)
- InChIKey
- WJCNZQLZVWNLKY-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


