| Synonyms |
Tiagabina; Tiagabina [INN-Spanish]; Tiagabine (INN); Tiagabine [INN:BAN]; Tiagabine [INN]; Tiagabinum; Tiagabinum [INN-Latin]; Gabatril; Gabitril; Gabitril (TN); NO 329; NO-328; NO050328; Z80I64HMNP; tiagabine; (-)-(R)-1-(4,4-Bis(3-methyl-2-thienyl)-3-butenyl)nipecotic acid; (R)-1-(4,4-Bis(3-methylthiophen-2-yl)but-3-en-1-yl)piperidine-3-carboxylic acid; (R)-Tiagabine; 115103-54-3; A-70569-1; ABBOTT-70569-1; ABT-569; Abbott-70569; CHEBI:9586; CHEMBL1027; NO328; UNII-Z80I64HMNP
|
| Cross-matching ID |
- PubChem CID
- 60648
- PubChem SID
-
9706
; 7980788
; 8186978
; 14804309
; 14926800
; 26758048
; 43118012
; 46505560
; 48416622
; 50015636
; 50510996
; 53787330
; 92723327
; 93166176
; 96025272
; 103276854
; 103946490
; 104321287
; 117542112
; 126592912
; 126617580
; 128891680
; 134337484
; 135016989
; 137002486
; 142970946
; 160847730
; 160964245
; 163418753
; 163850077
; 164814870
; 172858819
; 175268881
; 178101399
; 178101520
; 179116918
; 184545901
; 210279817
; 210282140
; 223441308
; 223554966
; 223655564
; 224165587
; 226421082
; 251916677
; 251917916
; 251970975
; 252357544
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0ED7U
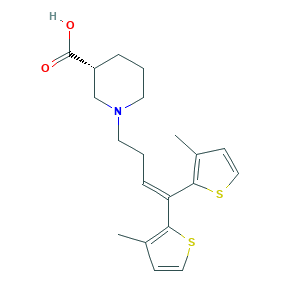
- Formula
- C20H25NO2S2
- Canonical SMILES
- CC1=C(SC=C1)C(=CCCN2CCCC(C2)C(=O)O)C3=C(C=CS3)C
- InChI
- 1S/C20H25NO2S2/c1-14-7-11-24-18(14)17(19-15(2)8-12-25-19)6-4-10-21-9-3-5-16(13-21)20(22)23/h6-8,11-12,16H,3-5,9-10,13H2,1-2H3,(H,22,23)/t16-/m1/s1
- InChIKey
- PBJUNZJWGZTSKL-MRXNPFEDSA-N
|