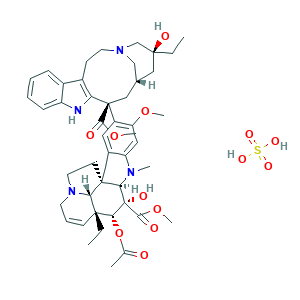
| Synonyms |
Vinblastine sulfate; Vinblastine sulfate [USAN:JAN]; Vinblastine sulphate; Vinblastine, hydrate; Vincaleucoblastine sulfate; Vincaleukoblastine sulfate; Vincaleukoblastine sulfate (1:1) (salt); Vincaleukoblastine sulfate salt; Vincaleukoblastine, sulfate; Vincaleukoblastine, sulfate (1:1) (salt); Vinblastin; Vinblastina; Vinblastina [DCIT]; Vinblastine [INN:BAN]; Vinblastinum; Vinblastinum [INN-Latin]; Vincaleucoblastin; Vincaleukoblastine; Vincoblastine; Vinblastine; (2ALPHA,2'BETA,3BETA,4ALPHA,5BETA)-VINCALEUKOBLASTINE; 29060-LE; 5V9KLZ54CY; 865-21-4; C46H58N4O9; CCRIS 9002; CHEMBL159; EINECS 212-734-0; HSDB 3263; NCI-C04842; NDC 0002-1452-01; NSC 47842; Nincaluicolflastine; Rozevin; UNII-5V9KLZ54CY; VR-8; Velban; Velbe; 143-67-9; 29060 LE; AI3-52943; Belvan, VLB; C46H58N4O9.H2O4S; CCRIS 2584; CHEBI:9984; EINECS 205-606-0; EXAL; Exal (TN); MFCD00082457; MFCD08706468; NSC 49842; NSC-49842; NSC49842; Rozevin sulfate; VLB monosulfate; Velban (TN); Vinblastine 5
|
| Cross-matching ID |
- PubChem CID
- 241902
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0W9MM
- Formula
- C46H60N4O13S
- Canonical SMILES
- CCC1(CC2CC(C3=C(CCN(C2)C1)C4=CC=CC=C4N3)(C5=C(C=C6C(=C5)C78CCN9C7C(C=CC9)(C(C(C8N6C)(C(=O)OC)O)OC(=O)C)CC)OC)C(=O)OC)O.OS(=O)(=O)O
- InChI
- 1S/C46H58N4O9.H2O4S/c1-8-42(54)23-28-24-45(40(52)57-6,36-30(15-19-49(25-28)26-42)29-13-10-11-14-33(29)47-36)32-21-31-34(22-35(32)56-5)48(4)38-44(31)17-20-50-18-12-16-43(9-2,37(44)50)39(59-27(3)51)46(38,55)41(53)58-7;1-5(2,3)4/h10-14,16,21-22,28,37-39,47,54-55H,8-9,15,17-20,23-26H2,1-7H3;(H2,1,2,3,4)/t28-,37+,38-,39-,42+,43-,44-,45+,46+;/m1./s1
- InChIKey
- KDQAABAKXDWYSZ-JKDPCDLQSA-N
|