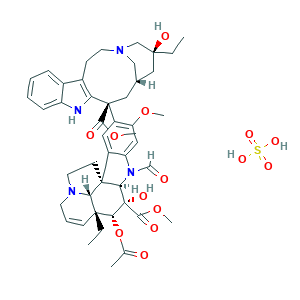
| Synonyms |
Vincristine sulfate; Vincristine sulphate; Vincristinsulfat [German]; Vincrisul; 2068-78-2; 22-oxovincaleukoblastine sulfate; AI3-52944; Alkaloid extracted from Vinca rosea Linn; CCRIS 2583; CHEBI:79401; EINECS 218-190-0; Leurocristine sulfate (1:1) (salt); Leurocristine, sulfate (1:1) (salt); Lilly 37231; MLS002702994; NSC 67574; NSC67574; Oncovin; Onkovin; VCR sulfate; Vincaleukoblastine, 22-oxo-, sulfate (1:1) (salt); Vincasar PFS
|
| Cross-matching ID |
- PubChem CID
- 249332
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D09QVV
- Formula
- C46H58N4O14S
- Canonical SMILES
- CCC1(CC2CC(C3=C(CCN(C2)C1)C4=CC=CC=C4N3)(C5=C(C=C6C(=C5)C78CCN9C7C(C=CC9)(C(C(C8N6C=O)(C(=O)OC)O)OC(=O)C)CC)OC)C(=O)OC)O.OS(=O)(=O)O
- InChI
- 1S/C46H56N4O10.H2O4S/c1-7-42(55)22-28-23-45(40(53)58-5,36-30(14-18-48(24-28)25-42)29-12-9-10-13-33(29)47-36)32-20-31-34(21-35(32)57-4)50(26-51)38-44(31)16-19-49-17-11-15-43(8-2,37(44)49)39(60-27(3)52)46(38,56)41(54)59-6;1-5(2,3)4/h9-13,15,20-21,26,28,37-39,47,55-56H,7-8,14,16-19,22-25H2,1-6H3;(H2,1,2,3,4)/t28-,37+,38-,39-,42+,43-,44-,45+,46+;/m1./s1
- InChIKey
- AQTQHPDCURKLKT-JKDPCDLQSA-N
|