| General Information of Drug (ID:
DR1697) |
| Drug Name |
AY-27,255
|
| Synonyms |
Vinpocetine; Apovincaminate d'ethyle [French]; Apovincaminic acid ethyl ester; Bravinton; Cavinton; Ceractin; Ethyl (+)-apovincaminate; Ethyl (+)-cis-apovincaminate; Ethyl apovincamin-22-oate; Ethyl apovincaminate; RGH 4405; RGH-4405; TCV-3B; Vinpocetinum [INN-Latin]; cis-Apovincaminic acid ethyl ester; vinpocetine; (+)-Apovincaminic acid ethyl ester; (+)-cis-Apovincaminic acid ethyl ester; 3-alpha,16-alpha-Apovincaminic acid ethyl ester; 42971-09-5; AY 27,255; EINECS 256-028-0; Eburnamenine-14-carboxylic acid ethyl ester; UNII-543512OBTC
|
| Indication |
Haemorrhagic stroke
[ICD11: 8B20]
|
Phase 2/3
|
[1]
|
| Structure |
|
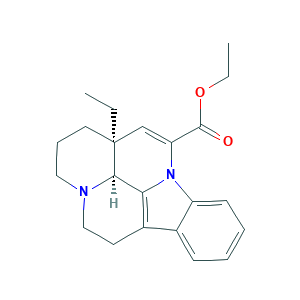 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
350.5 |
Topological Polar Surface Area |
34.5 |
| Heavy Atom Count |
26 |
Rotatable Bond Count |
4 |
| Hydrogen Bond Donor Count |
0 |
Hydrogen Bond Acceptor Count |
3 |
| Cross-matching ID |
- PubChem CID
- 443955
- PubChem SID
-
855619
; 3727081
; 7848434
; 7980299
; 10299443
; 10321872
; 11341598
; 11361781
; 11364294
; 11366856
; 11369418
; 11371881
; 11374568
; 11377580
; 11466296
; 11467416
; 11485054
; 11486071
; 11487183
; 11489345
; 11490776
; 11492889
; 11495214
; 14802886
; 14925387
; 17405811
; 24278182
; 26612408
; 26680355
; 26759739
; 36887098
; 47216903
; 47365339
; 47440398
; 47810892
; 47959906
; 48334637
; 48334638
; 48416699
; 48483328
; 49698412
; 49964760
; 50103960
; 50103961
; 50103962
; 50103963
; 50113284
; 53778363
; 53790458
; 56424092
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D06BCB
- Formula
- C22H26N2O2
- Canonical SMILES
- CCC12CCCN3C1C4=C(CC3)C5=CC=CC=C5N4C(=C2)C(=O)OCC
- InChI
- 1S/C22H26N2O2/c1-3-22-11-7-12-23-13-10-16-15-8-5-6-9-17(15)24(19(16)20(22)23)18(14-22)21(25)26-4-2/h5-6,8-9,14,20H,3-4,7,10-13H2,1-2H3/t20-,22+/m1/s1
- InChIKey
- DDNCQMVWWZOMLN-IRLDBZIGSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


