| General Information of Drug (ID:
DR1720) |
| Drug Name |
Ximelagatran
|
| Prodrug Info |
Ximelagatran is the prodrug of OH-melagatran
|
| Synonyms |
Ximelagatran; Ximelagatran [USAN:INN]; Glycine, N-((1)1-cyclohexyl-2-((2)-((((4-(amino(hydroxyimino)methyl)phenyl)methyl)amino)carbonyl)-1-azetidinyl)2-oxoethyl)-, ethyl ester; Exanta; Exarta; 192939-46-1; Glycine, N-((1R)-1-cyclohexyl-2-((2S)-2-((((4-(hydroxyamino)iminomethyl)phenyl)methyl)amino)carbonyl)-1-azetidinyl)-2-oxoethyl)-, ethyl ester; H 376-95; H 376/95; H 37695; NCGC00183598-01
|
| Indication |
Coagulation defect
[ICD11: 3B10]
|
Phase 4
|
[1]
|
| Structure |
|
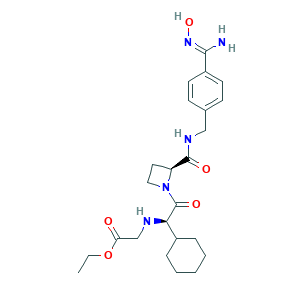 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
473.6 |
Topological Polar Surface Area |
146 |
| Heavy Atom Count |
34 |
Rotatable Bond Count |
11 |
| Hydrogen Bond Donor Count |
4 |
Hydrogen Bond Acceptor Count |
7 |
| Cross-matching ID |
- PubChem CID
- 9574101
- PubChem SID
-
7849043
; 12015148
; 14785299
; 14858738
; 14883129
; 14883130
; 23997623
; 44712163
; 46509040
; 49973459
; 57372958
; 79267685
; 87225336
; 124894453
; 134338458
; 135106952
; 137002923
; 137726685
; 140950577
; 142578696
; 144207182
; 160645186
; 160967831
; 164235510
; 164766173
; 180370510
; 226396762
; 226396763
; 233586233
; 251971104
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0JQ7Y
- Formula
- C24H35N5O5
- Canonical SMILES
- CCOC(=O)CNC(C1CCCCC1)C(=O)N2CCC2C(=O)NCC3=CC=C(C=C3)C(=NO)N
- InChI
- 1S/C24H35N5O5/c1-2-34-20(30)15-26-21(17-6-4-3-5-7-17)24(32)29-13-12-19(29)23(31)27-14-16-8-10-18(11-9-16)22(25)28-33/h8-11,17,19,21,26,33H,2-7,12-15H2,1H3,(H2,25,28)(H,27,31)/t19-,21+/m0/s1
- InChIKey
- ZXIBCJHYVWYIKI-PZJWPPBQSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


