| General Information of Drug (ID:
DR1752) |
| Drug Name |
Ethionamide
|
| Synonyms |
CHEBI:31536; DRG-0208; FTC-(-); HSDB 7337; UNII-G70B4ETF4S; dOTFC; Emtricitabine; Emtritabine; Emtriva; Ethimide; Ethina; Ethinamide; Ethioniamide; Ethylisothiamide; Ethyonomide; Etimid; Etiocidan; Etionamid; Etioniamid; Etionid; Etionizin; Etionizina; Etionizine; Fatoliamid; Iridocin; Iridozin; Isothin; Isotiamida; Itiocide; Nicotion; Nizotin; Racivir; Rigenicid; Sertinon; Teberus; Thianid; Thianide; Thioamide; Aetina; Aetiva; Amidazin; Amidazine; Coviracil; Thioniden; Tianid; Trecator; Trecator-SC; Trekator; Trescatyl; Trescazide; Tubenamide; Tubermin; Tuberoid; Tuberoson; ethionamide; (-)-2'-Deoxy-5-fluoro-3'-thiacytidine; (-)-FTC; 143491-54-7; 143491-57-0; 2',3',5-FTC; 2'-Deoxy-5-fluoro-3'-oxa-4'-thiocytidine; 2'-Deoxy-5-fluoro-3'-thiacytidine; 2-Ethylthioisonicotinamide; 2-FTC; 2-ethylpyridine-4-carbothioamide; 3'-Thia-2'.3'-dideoxy-5-fluorocytidine; 4-amino-5-fluoro-1-((2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)pyrimidin-2(1H)-one; 524W91; 536-33-4; Atina; BW 1592; BW 524W91; BW-524W91; BW524W91
|
| Indication |
Human immunodeficiency virus infection
[ICD11: 1C60]
|
Approved
|
[]
|
| Structure |
|
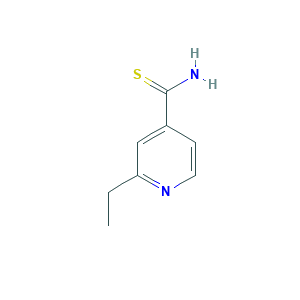 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
166.25 |
Topological Polar Surface Area |
71 |
| Heavy Atom Count |
11 |
Rotatable Bond Count |
2 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
2 |
| Cross-matching ID |
- PubChem CID
- 2761171
- PubChem SID
-
9867
; 138261
; 602897
; 3189207
; 5235093
; 7847657
; 7979199
; 8149340
; 8815239
; 10321785
; 10525861
; 11112402
; 11335320
; 11360559
; 11363976
; 11366538
; 11369100
; 11371336
; 11374352
; 11377262
; 11461531
; 11466554
; 11467674
; 11484823
; 11486168
; 11488810
; 11490244
; 11492362
; 11494896
; 11532957
; 15413224
; 17389895
; 24894350
; 26611740
; 26679347
; 26747295
; 26747296
; 26752898
; 39176212
; 46506077
; 47365040
; 47515175
; 47515176
; 47588853
; 47959584
; 48184860
; 48334345
; 48413732
; 48415974
; 48423391
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0P0HB
- Formula
- C8H10N2S
- Canonical SMILES
- CCC1=NC=CC(=C1)C(=S)N
- InChI
- 1S/C8H10N2S/c1-2-7-5-6(8(9)11)3-4-10-7/h3-5H,2H2,1H3,(H2,9,11)
- InChIKey
- AEOCXXJPGCBFJA-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


