| General Information of Drug (ID:
DR1766) |
| Drug Name |
Carprofen
|
| Synonyms |
Carprofene; Carprofene [INN-French]; Carprofeno; Carprofeno [INN-Spanish]; Carprofeno [Spanish]; Carprofenum; Carprofenum [INN-Latin]; Imadyl; Ridamyl; Rimadyl; Ro 20-5720; Ro 20-5720/000; carprofen; (+-)-6-Chloro-alpha-methylcarbazole-2-acetic acid; 2-(6-Chloro-9H-carbazol-2-yl)propanoic acid; 53716-49-7; 6-Chloro-alpha-methyl-9H-carbazole-2-acetic acid; 6-Chloro-alpha-methylcarbazole-2-acetic acid; BRN 0487098; C 5720; C15H12ClNO2; CCRIS 3507; CHEBI:364453; CHEMBL1316; EINECS 258-712-4; MFCD00079028; NSC 297935
|
| Indication |
Anaesthesia
[ICD11: 8E22]
|
Approved
|
[1]
|
| Structure |
|
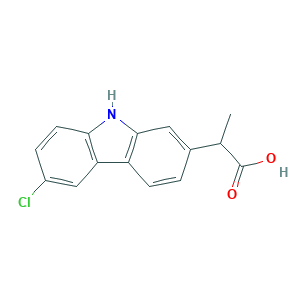 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
273.71 |
Topological Polar Surface Area |
53.1 |
| Heavy Atom Count |
19 |
Rotatable Bond Count |
2 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
2 |
| Cross-matching ID |
- PubChem CID
- 2581
- PubChem SID
-
147404
; 224736
; 7387300
; 8151706
; 11372077
; 11374733
; 11484848
; 11489052
; 11490754
; 11492852
; 12013355
; 14824217
; 17397550
; 24860673
; 25623932
; 26612250
; 26679932
; 26748565
; 26748566
; 29221741
; 46505357
; 48110633
; 49901603
; 49901604
; 50085988
; 51138619
; 57321383
; 57648952
; 85174420
; 85556037
; 92124308
; 92307546
; 92719046
; 99215020
; 103159169
; 103409544
; 103994678
; 104301004
; 117843310
; 124489038
; 124636988
; 124801035
; 125324613
; 125352521
; 126621128
; 126653073
; 126670260
; 128971698
; 131317931
; 131813290
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0IT2X
- Formula
- C15H12ClNO2
- Canonical SMILES
- CC(C1=CC2=C(C=C1)C3=C(N2)C=CC(=C3)Cl)C(=O)O
- InChI
- 1S/C15H12ClNO2/c1-8(15(18)19)9-2-4-11-12-7-10(16)3-5-13(12)17-14(11)6-9/h2-8,17H,1H3,(H,18,19)
- InChIKey
- PUXBGTOOZJQSKH-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


